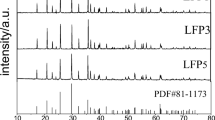
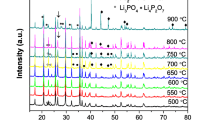
Abstract
LiFePO4/C composites were successfully prepared by a solid-state reaction in order to compare conventional heat treatment and microwave-assisted synthesis at different times of sintering. Microwave-assisted synthesis is interesting due to the fact that energy and inert gas consumption can be greatly reduced with respect to the conventional treatment, resulting in a cheaper synthesis method. The relationship between particle morphology and crystal structure using the composite synthesis was characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), and X-ray diffraction (XRD) with refinements of the crystal structures carried out by the Rietveld method. In addition, the electrochemical performances were evaluated using constant current charge/discharge tests, cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS). It was observed that the samples prepared by microwave heating had a better electrochemical behavior than those prepared in a conventional furnace. Also, in general, a higher sintering time improved the electrochemical behavior, but with increased particle sizes, and consequently, a decreased specific capacity.
Graphical Abstract








Similar content being viewed by others
References
Whittingham MS (2004) Lithium batteries and cathode materials. Chem Rev 104:4271–4301. doi:10.1021/cr020731c
Zhang WJ (2011) Structure and performance of LiFePO4 cathode materials: a review. J Power Sources 196:2962–2970. doi:10.1016/j.jpowsour.2010.11.113
Winter M, Brodd RJ (2004) What are batteries, fuel cells, and supercapacitors ? Chem Rev 104:4245–4270. doi:10.1021/cr020730k
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc 144:1188–1194. doi:10.1684/agr.2014.0700
Peng P, Jiang F (2016) Thermal safety of lithium-ion batteries with various cathode materials: a numerical study. Int J Heat Mass Transf 103:1008–1016. doi:10.1016/j.ijheatmasstransfer.2016.07.088
Zhou F, Kang K, Maxisch T et al (2004) The electronic structure and band gap of LiFePO4 and LiMnPO4. Solid State Commun 132:181–186. doi:10.1016/j.ssc.2004.07.055
Inagaki M (2012) Carbon coating for enhancing the functionalities of materials. Carbon N Y 50:3247–3266. doi:10.1016/j.carbon.2011.11.045
Feng J, Wang Y (2016) High-rate and ultralong cycle-life LiFePO4 nanocrystals coated by boron-doped carbon as positive electrode for lithium-ion batteries. Appl Surf Sci 390:481–488. doi:10.1016/j.apsusc.2016.08.066
Wang J, Sun X (2015) Olivine LiFePO4: the remaining challenges for future energy storage. Energy Environ Sci 8:1110–1138. doi:10.1039/C4EE04016C
Hsieh C-T, Liu J-R, Juang R-S et al (2015) Microwave synthesis of copper network onto lithium iron phosphate cathode materials for improved electrochemical performance. Mater Chem Phys 153:103–109. doi:10.1016/j.matchemphys.2014.12.040
Naik A, Zhou J, Gao C et al (2016) Rapid and facile synthesis of Mn doped porous LiFePO4/C from iron carbonyl complex. J Energy Inst 89:21–29. doi:10.1016/j.joei.2015.01.013
Jugović D, Uskoković D (2009) A review of recent developments in the synthesis procedures of lithium iron phosphate powders. J Power Sources 190:538–544. doi:10.1016/j.jpowsour.2009.01.074
Wang Z, Guo H, Yan P (2014) A rapid microwave heating route to synthesize graphene modified LiFePO4/C nanocomposite for rechargeable lithium-ion batteries. Ceram Int 40:15801–15806. doi:10.1016/j.ceramint.2014.07.106
Yu F, Zhang L, Zhu M et al (2014) Overwhelming microwave irradiation assisted synthesis of olivine-structured LiMPO4 (M = Fe, Mn, Co and Ni) for Li-ion batteries. Nano Energy 3:64–79. doi:10.1016/j.nanoen.2013.10.011
Wang L, Liang GC, Ou XQ et al (2009) Effect of synthesis temperature on the properties of LiFePO4/C composites prepared by carbothermal reduction. J Power Sources 189:423–428. doi:10.1016/j.jpowsour.2008.07.032
Rietveld HM (1969) A profile refinement method for nuclear and magnetic structures. J Appl Crystallogr 2:65–71. doi:10.1107/S0021889869006558
Rodríguez-Carvajal J (1993) Recent advances in magnetic structure determination by neutron powder diffraction. Phys B Condens Matter 192:55–69. doi:10.1016/0921-4526(93)90108-I
Zhu Y, Tang S, Shi H, Hu H (2014) Synthesis of FePO4∙×H2O for fabricating submicrometer structured LiFePO4/C by a co-precipitation method. Ceram Int 40:2685–2690. doi:10.1016/j.ceramint.2013.10.055
Kim CW, Lee MH, Jeong WT, Lee KS (2005) Synthesis of olivine LiFePO4 cathode materials by mechanical alloying using iron(III) raw material. J Power Sources 146:534–538. doi:10.1016/j.jpowsour.2005.03.058
Kim CW, Park JS, Lee KS (2006) Effect of Fe2P on the electron conductivity and electrochemical performance of LiFePO4 synthesized by mechanical alloying using Fe3+ raw material. J Power Sources 163:144–150. doi:10.1016/j.jpowsour.2006.02.071
Yamada A, Chung SC, Hinokuma K (2001) Optimized LiFePO4 for lithium battery cathodes. J Electrochem Soc 148:A224–A229. doi:10.1149/1.1348257
Hu YS, Guo YG, Dominko R et al (2007) Improved electrode performance of porous LiFePO4 using RuO2 as an oxidic nanoscale interconnect. Adv Mater 19:1963–1966. doi:10.1002/adma.200700697
Yu F, Zhang J, Yang Y, Song G (2010) Porous micro-spherical aggregates of LiFePO4/C nanocomposites: a novel and simple template-free concept and synthesis via sol-gel-spray drying method. J Power Sources 195:6873–6878. doi:10.1016/j.jpowsour.2010.01.042
Zhang M, Liu R, Feng F et al (2015) Etching preparation of (010)-defective LiFePO4 platelets to visualize the one-dimensional migration of Li+ ions. J Phys Chem C 119:12149–12156. doi:10.1021/acs.jpcc.5b02270
Delacourt C, Laffont L, Bouchet R et al (2005) Toward understanding of electrical limitations (electronic, ionic) in LiMPO4 (M = Fe, Mn) electrode materials. J Electrochem Soc 152:A913. doi:10.1149/1.1884787
Wang J, Sun X (2012) Understanding and recent development of carbon coating on LiFePO4 cathode materials for lithium-ion batteries. Energy Environ Sci 5:5163–5185. doi:10.1039/C1EE01263K
Bard AJ, Faulkner LR, York N et al (1944) Electrochemical methods fundamentals and applications. Electrochem I Faulkner, Larry R. doi:10.1016/B978-0-12-381373-2.00056-9
Yu DYW, Fietzek C, Weydanz W et al (2007) Study of LiFePO4 by cyclic voltammetry. J Electrochem Soc 154:A253. doi:10.1149/1.2434687
Morgan D, Van der Ven A, Ceder G (2004) Li conductivity in LixMPO4 (M = Mn, Fe Co, Ni) olivine materials. Electrochem Solid-State Lett 7:A30. doi:10.1149/1.1633511
Ouyang C, Shi S, Wang Z et al (2004) First-principles study of Li ion diffusion in LiFePO4. Phys Rev B 69:104303. doi:10.1103/PhysRevB.69.104303
Park CK, Bin Park S, Oh SH et al (2011) Li ion diffusivity and improved electrochemical performances of the carbon coated LiFePO4. Bull Korean Chem Soc 32:836–840. doi:10.5012/bkcs.2011.32.3.836
Prosini PP, Lisi M, Zane D, Pasquali M (2002) Determination of the chemical diffusion coefficient of lithium in LiFePO4. Solid State Ionics 148:45–51. doi:10.1016/S0167-2738(02)00134-0
Park M, Zhang X, Chung M et al (2010) A review of conduction phenomena in Li-ion batteries. J Power Sources 195:7904–7929. doi:10.1016/j.jpowsour.2010.06.060
Acknowledgements
We thank the financial support of the “CONICET UE Desarrollo de Baterías de Litio,” Argentina. We thank Dr. Paul Hobson, native speaker, for revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Calderón, C.A., Thomas, J.E., Lener, G. et al. Electrochemical comparison of LiFePO4 synthesized by a solid-state method using either microwave heating or a tube furnace. J Appl Electrochem 47, 1179–1188 (2017). https://doi.org/10.1007/s10800-017-1111-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-017-1111-0