Abstract
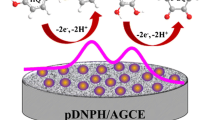
In this work, we present a comparison among three glassy carbon electrodes modified by Co-porphyrin, ortho-phenylenediamine, or both simultaneously. This comparison shows the differences among the electrochemical behavior, morphological characteristics and electrocatalytic behavior toward the sulfite oxidation of these electrodes. The electrode modified by Co-porphyrin, ortho-phenylenediamine and copolymer has been investigated in detail for the comparision of electrocatalytic activity towards the sulfite oxidation. In the case of the glassy carbon-modified electrodes, the presence of the copolymer enhances the electrocatalytic performance of the modified electrodes in spite of the non-catalytic response (compared to the bare glassy carbon) of both homopolymer-modified electrodes toward the oxidation of sulfite. Additionally, the oxidation of sulfite extracted from red wine is shown. The copolymer-modified electrode is capable of oxidizing the extracted free sulfite in a 0.02 M NaOH solution. Through the addition of standards method, a concentration of free sulfite in a Chilean red wine sample was determined to be 44 ppm.










Similar content being viewed by others
References
Dai H-P, Wu Q-H, Sun S-G, Shiu K-K (1998) Electrochemical quartz crystal microbalance studies on the electropolymerization processes of ortho-phenylenediamine in sulfuric acid solutions. J Electroanal Chem 456(1–2):47
Ogura K, Kokura M, Yano J, Shiigi H (1995) Spectroscopic and scanning tunneling microscopic characterization of virgin and recast films of electrochemically prepared poly(o-phenylenediamine). Electrochim Acta 40(17):2707
Wu LL, Luo J, Lin ZH (1996) Spectroelectrochemical studies of poly-o-phenylenediamine. Part 1. In situ resonance Raman spectroscopy. J Electroanal Chem 417(1–2):53
Maiyalagan T (2008) Electrochemical synthesis, characterization and electro-oxidation of methanol on platinum nanoparticles supported poly(o-phenylenediamine) nanotubes. J Power Sources 179(2):443
Martinusz K, Láng G, Inzelt G (1997) Impedance analysis of poly(o-phenylenediamine) electrodes. J Electroanal Chem 433(1):1–8
Martinusz K, Czirok E, Inzelt G (1994) Studies of the formation and redox transformation of poly(o-phenylenediamine) films using a quartz crystal microbalance. J Electroanal Chem 379(1–2):437
White BA, Murray RW (1985) Electroactive porphyrin films from electropolymerized metallotetra (o-aminophenyl) porphyrins. J Electroanal Chem 189:345
Peljo P, Rauhala T, Murtomäki L, Kallio T, Kontturi K (2011) Oxygen reduction at a water-1,2-dichlorobenzene interface catalyzed by cobalt tetraphenyl porphyrine––a fuel cell approach. Int J Hydrog Energy 36(16):10033
Riquelme MA, Isaacs M, Lucero M, Trollund E, Aguirre MJ, Canales J (2003) Electrocatalytic reduction of carbon dioxide at polymeric Cobalt tetra (3-Aminophenyl) porphyrin glassy carbon-modified electrodes. J Chil Chem Soc 48(2):2
Armijo F, Isaacs M, Ramírez G, Trollund E, Canales MJ, Aguirre MJ (2004) Electrocatalytic reduction of nitrate and electrical characterization of modified electrodes with polytetraminophenylporphyrins-modified electrodes. J Electroanal Chem 566(2):315
Trollund E, Ardiles P, Aguirre MJ, Biaggio SR, Rocha-Filho RC (2000) Spectroelectrochemical and electrical characterization of poly(cobal-tetraminophthalocyanine) modified electrodes: electrocatalytic oxidation of hydrazine. Polyhedron 19:2303–2312
Isaacs M, Canales JC, Riquelme A, Lucero M, Aguirre MJ, Costamagna J (2003) Contribution of the ligand on the electroreduction of CO2 catalized by a cobalt(II) macrocyclic complex. J Coord Chem 56(14):1193
Ramírez G, Lucero M, Riquelme A, Villagrán M, Costamagna J, Trollund E, Aguirre MJ (2004) A supramolecular Cobalt-porphyrin-modified electrode toward the electroreduction of CO2. J Coord Chem 57:249
Griveau S, Albin V, Pauporté T, Bedioui F, Zagal J (2002) Comparative study of electropolymerized cobalt porphyrin and phthalocyanine based films for the electrochemical activation of thiols. J Mater Chem 12(2):225–232
Lucero M, Riquelme M, Ramírez G, Goya MC, González Orive A, Hernández Creus A, Arévalo MC, Aguirre MJ (2012) A new modified electrode with a copolymer of aniline/Fe(III)-Tetrakis(Para-Aminophenyl)porphyrin: test of its electrocatalytic activity toward the reduction of molecular oxygen and oxidation of ascorbic acid and sulfite ion. Int J Electrochem Sci 7:234–250
Chen WC, Wen TC, Gopalan A (2001) Electrochemical and spectroelectrochemical evidences for copolymer formation between 2-aminodiphenylamine and aniline. J Electrochem Soc 148(11):E427–E434
Yamamoto K, Tanaichi D (2000) A self-doped oligoaniline with two stable redox couples in a wide pH range. Macromol Chem Phys 201(1):649
Heras JY, Giacobone AFF, Battaglini F (2007) Ascorbate amperometric determination using conducting copolymers from aniline and N-(3-propane sulfonic acid)aniline. Talanta 71(4):1684–1689
Latonen RM, Kvarnstöm C, Ivaska A (1999) Electrochemical synthesis of a copolymer of poly(3-octylthiophene) and poly(paraphenylene). Electrochim Acta 44(12):1933–1943
Chen-Yang YW, Li JL, Wu TL, Wang WS, Hon TF (2004) Electropolymerization and electrochemical properties of (N-hydroxyalkyl)pyrrole/pyrrole copolymers. Electrochim Acta 49(12):2031–2040
Tang H, Kitani A, Ito S (1997) Electrochemical copolymerization of aniline and aniline-2,5-disulfonic acid. Electrochim Acta 42(23–24):3421–3428
Bilal S, Holze R (2007) In situ UV–vis spectroelectrochemistry of poly(o-phenylenediamine-co-m-toluidine). Electrochim Acta 52:5346–5356
Arce R, del Río R, Ruíz-León D, Vélez J, Isaacs M, del Valle MA, Aguirre MJ (2012) Evidence for the formation of a copolymer by simultaneous electropolymerization of p-tetraaminophenyl porphyrin Cobalt(II) and o-phenylenediamine on glassy carbon. Int J Electrochem Sci 7:11596–11608
Losito I, De Giglio E, Cioffi N, Malitesta C (2001) Spectroscopic investigation on polymer films obtained by oxidation of o-phenylenediamine on platinum electrodes at different pHs. J Mater Chem 11:1812–1817
Zhou H, Yang W, Sun C (2004) Amperometric sulfite sensor based on multiwalled carbon nanotubes/ferrocene-branched chitosan composites. Talanta 77(1):366–371
Tolmachev Y, Scherson D (2004) The electrochemical oxidation of sulfite on gold: uV-Vis reflectance spectroscopy at a rotating disk electrode. Electrochim Acta 49(8):1315–1319
Carballo R, Campo Dall’ Orto V, Lo Balbo A, Rezzano I (2003) Determination of sulfite by flow injection analysis using a poly[Ni-(protoporphyrin IX)] chemically modified electrode. Sens Actuat B 88(2):155–161
David R (2000) Hanbook of chemistry and physics, 73a edn. CRC Press, Inc., Florida
Arce R, Tesis de Doctorado, Copolímeros de para-tetraaminofenilporfirina de cobalto(II) y orto-fenilendiamina como recubrimientos de electrodos: propiedades electrocatalíticas versus condiciones de electrosíntesis (2012) Universidad de Santiago de Chile, Chile
Lucero M, Ramírez G, Riquelme A, Azócar I, Isaacs M, Armiijo F, Forster JE, Trollund E, Aguirre MJ, Lexa D (2004) Electrocatalytic oxidation of sulfite at polymeric iron tetra (4-aminophenyl) porphyrin—modified electrode. J Mol Catal A: Chem 221(1–2):71
Arce R, Márquez P, Herrera F, Aguirre MJ, Romero J (2013) Sulfite oxidation mediated by ortho-phenylenediamine/Co(II)-tetrakis(para-aminophenyl)porphyrin copolymers in acid medium. J Chil Chem Soc 58(4):1982–1985
Karaman R, Jeon S, Almarson O, Bruice TC (1992) Symmetrical and unsymmetrical quadruply aza bridged closely interspaced cofacial bis-5,10,15,20-tetraphenylporphyrins 3. Intraplanar distance, 1H NMR chemical shifts and the catalysis of the electrochemical reduction of oxygen. J Am Chem Soc 114:4899
A. Plaza, J. Romero, W. Silva, E. Morales, A. Torres, and M. J. Aguirre, (2013) New metodology for extraction and quantification of the sulfite content from wine by means of a membrane contactor operation. Food Sci and Technol Int. In Press
Hasanoğlu A, Romero J, Plaza A, Silva W (2013) Gas-filled membrane absorption processes: a review of three different applications to describe the mass transfer by means of a unified approach. Desalination 51:5649–5663. doi:10.1080/19443994.2013.769603
http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?an=21:2.0.1.1.2.71.4.1 (access in June 2014)
Montes C, Vélez JH, Ramírez G, Isaacs M, Arce R, Aguirre MJ (2012) Critical comparison between modified Monier-Williams and electrochemical methods to determine sulfite in aqueous solutions. Sci World J. doi:10.1100/2012/168148
Acknowledgments
Financial support from Fondecyt-Postdoctoral 3130594 and 1120071 Fondecyt-Regular projects. This study has been supported by Project ICM-P10-003-F, CILIS, granted by Fondo de Innovación para la Competitividad, del Ministerio de Economía, Fomento y Turismo, Chile.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arce, R., Romero, J. & Aguirre, M.J. A glassy carbon electrode modified by a copolymer of Co-tetrakis (para-aminophenyl)porphyrin and ortho-phenylenediamine. Characterization and electrocatalytic sulfite oxidation behavior of a basic extract from red wine. J Appl Electrochem 44, 1361–1369 (2014). https://doi.org/10.1007/s10800-014-0750-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-014-0750-7