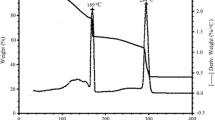
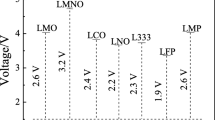
Abstract
Lithium transition metal phosphates have the capability of improving cathode energy densities up to 800 Wh kg−1, a 27 % increase over conventional cathode active material energy densities. In this study, the effect of base-to-acid (NH4OH:H3PO4) stoichiometric conditions on the intrinsic reversible capacity of lithium cobalt phosphate (LiCoPO4) active material are investigated through microwave synthesis and electrochemical testing. Variation in solution pH results in an increase of 69 mAh g−1 in achievable capacity. X-ray diffraction results show highly crystalline LiCoPO4, with particle sizes ranging from 200 nm to greater than 1 μm based upon scanning electron microscopy. Electrochemical analysis with 1 M LiPF6 EC:EMC (1:2 v/v) provides the highest capacity over multiple cycles. A discharge capacity of 128 mAh g−1 (78 % of theoretical capacity) is achievable for intrinsic LiCoPO4 without further treatment (e.g., carbon coating) at an effective 0.1 C rate with a proper constant current–constant voltage step. Analysis of reported synthesis techniques shows that microwave synthesis yields the highest capacity for the intrinsic LiCoPO4 material to date.






Similar content being viewed by others
References
Endo M, Kim C, Nishimura K, Fujino T, Miyashita K (2000) Recent development of carbon materials for Li ion batteries. Carbon 38:183–197
Daniel C (2008) Materials and processing for lithium-ion batteries. JOM J Miner Met Mater Soc 60(9):43–48
Howell D (2009) Progress Report for Energy Storage Research and Development. U.S. Department of Energy, Washington, DC
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22(3):587–603. doi:10.1021/cm901452z
Wakihara M, Nakayama M, Goto S, Uchimoto Y, Kitajima Y (2004) Changes in electronic structure between cobalt and oxide ions of lithium cobalt phosphate as 4.8-V positive electrode material. Chem Mater 16(18):3399–3401. doi:10.1021/cm049230t
Shih HC, Chen YC, Chen JM, Hsu CH, Lee JJ, Lin TC, Yeh JW (2010) Electrochemical and structural studies of LiCo(1/3)Mn(1/3)Fe(1/3)PO(4) as a cathode material for lithium ion batteries. J Power Sources 195(19):6867–6872. doi:10.1016/j.jpowsour.2010.01.058
Lloris JM, Vicente CP, Tirado JL (2002) Improvement of the electrochemical performance of LiCoPO(4) 5 V material using a novel synthesis procedure. Electrochem Solid State Lett 5(10):A234–A237. doi:10.1149/1.1507941
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc 144(4):1188–1194
Dileo RA, Castiglia A, Ganter MJ, Rogers RE, Cress CD, Raffaelle RP, Landi BJ (2010) Enhanced capacity and rate capability of carbon nanotube based anodes with titanium contacts for lithium ion batteries. ACS Nano 4(10):6121–6131. doi:10.1021/nn1018494
DiLeo RA, Frisco S, Ganter MJ, Rogers RE, Raffaelle RP, Landi BJ (2011) Hybrid germanium nanoparticle-single-wall carbon nanotube free-standing anodes for lithium ion batteries. J Phys Chem C 115(45):22609–22614. doi:10.1021/jp205992w
DiLeo RA, Ganter MJ, Raffaelle RP, Landi BJ (2010) Germanium-single-wall carbon nanotube anodes for lithium ion batteries. J Mater Res 25(8):1441–1446. doi:10.1557/jmr.2010.0184
Landi BJ, Ganter MJ, Cress CD, DiLeo RA, Raffaelle RP (2009) Carbon nanotubes for lithium ion batteries. Energy Environ Sci 2(6):638–654. doi:10.1039/b904116h
Ganter MJ, DiLeo RA, Schauerman CM, Rogers RE, Rafaelle RP, Landi BJ (2011) Differential scanning calorimetry analysis of an enhanced LiNi0.8Co0.2O2 cathode with single wall carbon nanotube conductive additives. Electrochim Acta 56:7272–7277
Bramnik NN, Nikolowski K, Trots DM, Ehrenberg H (2008) Thermal stability of LiCoPO4 cathodes. Electrochem Solid State Lett 11(6):A89–A93. doi:10.1149/1.2894902
Okada S, Sawa S, Egashira M, Yamaki J, Tabuchi M, Kageyama H, Konishi T, Yoshino A (2001) Cathode properties of phospho-olivine LiMPO4 for lithium secondary batteries. J Power Sources 97–8:430–432
Bramnik NN, Nikolowski K, Baehtz C, Bramnik KG, Ehrenberg H (2007) Phase transitions occurring upon lithium insertion-extraction of LiCoPO4. Chem Mater 19(4):908–915. doi:10.1021/cm062246u
Koleva V, Zhecheva E, Stoyanova R (2010) Ordered olivine-type lithium–cobalt and lithium–nickel phosphates prepared by a new precursor method. Eur J Inorg Chem 26:4091–4099. doi:10.1002/ejic.201000400
Okada S, Ueno M, Uebou Y, Yamaki J (2005) Fluoride phosphate Li(2)COPO(4)F as a high-voltage cathode in Li-ion batteries. J Power Sources 146(1–2):565–569. doi:10.1016/j.jpowsour.2005.03.149
Ma JF, Huang XA, Wu PW, Hu YM, Dai JH, Zhu ZB, Chen HY, Wang HF (2005) Hydrothermal synthesis of LiCoPO4 cathode materials for rechargeable lithium ion batteries. Mater Lett 59(5):578–582. doi:10.1016/j.matlet.2004.10.049
Xia DG, Zhao YJ, Wang SJ, Zhao CS (2009) Synthesis and electrochemical performance of LiCoPO(4) micron-rods by dispersant-aided hydrothermal method for lithium ion batteries. Rare Met 28(2):117–121. doi:10.1007/s12598-009-0023-5
Poovizhi PN, Selladurai S (2011) Study of pristine and carbon-coated LiCoPO(4) olivine material synthesized by modified sol–gel method. Ionics 17(1):13–19. doi:10.1007/s11581-010-0496-0
Yang JS, Xu JJ (2006) Synthesis and characterization of carbon-coated lithium transition metal phosphates LiMPO4 (M = Fe, Mn, Co, Ni) prepared via a nonaqueous sol–gel route. J Electrochem Soc 153(4):A716–A723. doi:10.1149/1.2168410
Manthiram A, Murugan AV, Muraliganth T, Ferreira PJ (2009) Dimensionally modulated, single-crystalline LiMPO(4) (M = Mn, Fe, Co, and Ni) with nano-thumblike shapes for high-power energy storage. Inorg Chem 48(3):946–952. doi:10.1021/ic8015723
Badsar M, Edrissi M (2010) Synthesis and characterization of different nanostructures of cobalt phosphate. Mater Res Bull 45(9):1080–1084. doi:10.1016/j.materresbull.2010.06.022
Li HH, Jin J, Wei JP, Zhou Z, Yan J (2009) Fast synthesis of core–shell LiCoPO4/C nanocomposite via microwave heating and its electrochemical Li intercalation performances. Electrochem Commun 11(1):95–98. doi:10.1016/j.elecom.2008.10.025
Murugan AV, Muraliganth T, Manthiram A (2009) One-pot microwave-hydrothermal synthesis and characterization of carbon-coated LiMPO4 (M = Mn, Fe, and Co) cathodes. J Electrochem Soc 156(2):A79–A83. doi:10.1149/1.3028304
Liao LX, Zuo PJ, Ma YL, Chen XQ, An YX, Gao YZ, Yin GP (2012) Effects of temperature on charge/discharge behaviors of LiFePO4 cathode for Li-ion batteries. Electrochim Acta 60:269–273. doi:10.1016/j.electacta.2011.11.041
Doan TNL, Taniguchi I (2011) Cathode performance of LiMnPO(4)/C nanocomposites prepared by a combination of spray pyrolysis and wet ball-milling followed by heat treatment. J Power Sources 196(3):1399–1408. doi:10.1016/j.jpowsour.2010.08.067
Dimesso L, Forster C, Jaegermann W, Khanderi JP, Tempel H, Popp A, Engstler J, Schneider JJ, Sarapulova A, Mikhailova D, Schmitt LA, Oswald S, Ehrenberg H (2012) Developments in nanostructured LiMPO4 (M = Fe, Co, Ni, Mn) composites based on three dimensional carbon architecture. Chem Soc Rev 41(15):5068–5080. doi:10.1039/c2cs15320c
Martha SK, Haik O, Zinigrad E, Exnar I, Drezen T, Miners JH, Aurbach D (2011) On the thermal stability of olivine cathode materials for lithium-ion batteries. J Electrochem Soc 158(10):A1115–A1122. doi:10.1149/1.3622849
Ben Mayza A, Ramanathan M, Radhakrishnan R, Ha S, Ramani V, Prakash J, Zaghib K (2011) Thermal characterization of LiFePO4 cathode in lithium ion cells. ECS Trans 35(34):177–183. doi:10.1149/1.3654216
Zhou MJ, Zhao LW, Okada S, Yamaki J (2011) Thermal characteristics of a FeF3 cathode via conversion reaction in comparison with LiFePO4. J Power Sources 196(19):8110–8115. doi:10.1016/j.jpowsour.2011.05.042
Joachin H, Kaun TD, Zaghib K, Prakash J (2009) Electrochemical and thermal studies of carbon-coated LiFePO4 cathode. J Electrochem Soc 156(6):A401–A406. doi:10.1149/1.3106121
Jang IC, Lim HH, Lee SB, Karthikeyan K, Aravindan V, Kang KS, Yoon WS, Cho WI, Lee YS (2010) Preparation of LiCoPO4 and LiFePO4 coated LiCoPO4 materials with improved battery performance. J Alloy Compd 497(1–2):321–324. doi:10.1016/j.jallcom.2010.03.055
Acknowledgments
We acknowledge funding support from the Department of Energy (DE-FG36-08 GO88110) and RIT Office for Diversity & Inclusion. We also acknowledge financial support from the U.S. Government including a Grant from the Intelligence Community Postdoctoral Research Fellowship Program through funding from the Office of the Director of National Intelligence. R.A.D. acknowledges graduate student funding from a GAANN fellowship through the RIT Microsystems Engineering Ph.D. Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rogers, R.E., Clarke, G.M., Matthew, O.N. et al. Impact of microwave synthesis conditions on the rechargeable capacity of LiCoPO4 for lithium ion batteries. J Appl Electrochem 43, 271–278 (2013). https://doi.org/10.1007/s10800-012-0517-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-012-0517-y