Abstract
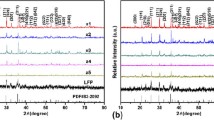
Lithium iron phosphate mixed with nano-sized acetylene black (LiFePO4-AB) was synthesized by a hydrothermal method and subsequent high-energy ball-milling process. Different contents of AB were added to improve the electronic conductivity of LiFePO4. The structural and morphological performance of LiFePO4-AB was investigated by X-ray diffraction (XRD), Raman spectroscopy, scanning electron microscope, and high-resolution transmission electron microscope. LiFePO4-AB/Li batteries were fabricated in an argon-filled glove box, and their electrochemical performance was analyzed by cyclic voltammetry and charge/discharge tests. The XRD results demonstrate that LiFePO4-AB has an olivine-type structure indexed to the orthorhombic Pnma space group. LiFePO4-AB/Li battery with 10 wt% AB shows the best high-rate discharge properties with the discharge capacities of 116 mAh g−1 at 1 C and 85 mAh g−1 at 10 C at room temperature.










Similar content being viewed by others
References
Tarascon J-M, Armand M (2001) Nature 414:359
Gu YX, Chen DR, Jiao XL (2005) J Phys Chem B 109:17901
Sauvage F, Tarascon J-M, Baudrin E (2007) J Phys Chem C 111:9264
Zheng HH, Zhang HC, Fu YB, Abe T, Ogumi Z (2005) J Phys Chem B 109:13676
Luo JY, Wang YG, Xiong HM, Xia YY (2007) Chem Mater 19:4791
Lee HC, Chang S-K, Goh E-Y, Jeong J-Y, Lee JH, Kim H-J, Cho J-J, Hong S-T (2008) Chem Mater 20:5
Jin B, Kim J-U, Gu H-B (2003) J Power Sources 117:148
Jayalakshmi M, Rao MM, Scholz F (2003) Langmuir 19:8403
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) J Electrochem Soc 144:1188
Hu YS, Guo YG, Dominko R, Gaberscek M, Jamnik J, Maier J (2007) Adv Mater 19:1963
Aldon L, Perea A, Womes M, Ionica-Bousquet CM, Jumas J-C (2010) J Solid State Chem 183:218
Wang Y, Wang J, Yang J, Nuli Y (2006) Adv Funct Mater 16:2135
Fisher CAJ, Prieto VMH, Islam MS (2008) Chem Mater 20:5907
Delacourt C, Poizot P, Morcrette M, Tarascon J-M, Masquelier C (2004) Chem Mater 16:93
Wang LN, Li ZC, Xu HJ, Zhang KL (2008) J Phys Chem C 112:308
Bramnik NN, Nikolowski K, Baehtz C, Bramnik KG, Ehrenberg H (2007) Chem Mater 19:908
Zaghib K, Mauger A, Goodenough JB, Gendron F, Julien CM (2007) Chem Mater 19:3740
Amine K, Yasuda H, Yamachi M (2000) Electrochem Solid State Lett 3:178
Islam MS, Driscoll DJ, Fisher CAJ, Slater PR (2005) Chem Mater 17:5085
Jin B, Jin EM, Park K-H, Gu H-B (2008) Electrochem Commun 10:1537
Molenda J, Stoklosa A, Bak T (1989) Solid State Ion 36:53
Shimakawas Y, Numata T, Tabuchi J (1997) J Solid State Chem 131:138
Gabrisch H, Wilcox JD, Doeff MM (2006) Electrochem Solid-State Lett 9:A360
Chung S-Y, Bloking JT, Chiang Y-M (2002) Nat Mater 1:123
Yamada A, Chung SC, Hinikuma K (2001) J Electrochem Soc 148:A224
Gibot P, Cabanas MC, Laffont L, Levasseur S, Carlach P, Hamelet S, Tarascon J-M, Masquelier C (2008) Nat Mater 7:741
Meethong N, Huang H-YS, Speakman SA, Carter WC, Chiang Y-M (2007) Adv Funct Mater 17:1115
Stevens R, Dodd JL, Kresch MG, Yazami R, Fultz B, Ellis B, Nazar LF (2006) J Phys Chem B 110:22732
Dominko R, Bele M, Goupil J-M, Gaberscek M, Hanzel D, Arcon I, Jamnik J (2007) Chem Mater 19:2960
Giorgetti M, Berrettoni M, Scaccia S, Passerini S (2006) Inorg Chem 45:2750
Franger S, Cras FL, Bourbon C, Rounault H (2002) Electrochem Solid-State Lett 5:A231
Jin B, Gu H-B (2008) Solid State Ion 178:1907
Sanchez MAE, Brito GES, Fantini MCA, Goya GF, Matos JR (2006) Solid State Ion 177:497
Arnold G, Garche J, Hemmer R, Strobele S, Volger C, Wohlfahrt-Mehrens M (2003) J Power Sources 119–121:247
Tuinstra F, Koenig JL (1970) J Chem Phys 53:1126
Sinha K, Menédez J (1990) Phys Rev B 41:10845
Palomares V, Goñi A, de Muro IGil, de Meatza I, Bengoechea M, Cantero I, Rojo T (2010) J Power Sources 195:7661
Acknowledgments
The authors acknowledge the financial supports from National Key Basic Research and Development Program of China (Grant No. 2010CB631001) and the China Postdoctoral Science Foundation Funded Project (Grant No. 20090451124). This research project also receives supporting funds from the second-stage Brain Korea 21.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, G., Jin, B., Sun, G. et al. Characteristics of lithium iron phosphate mixed with nano-sized acetylene black for rechargeable lithium-ion batteries. J Appl Electrochem 41, 99–106 (2011). https://doi.org/10.1007/s10800-010-0213-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-010-0213-8