Abstract
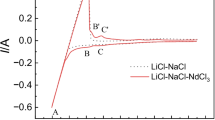
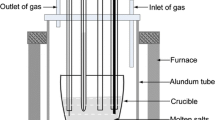
This study presents a novel electrochemical study on the codeposition of Mg, Li, and Mn on a molybdenum electrode in LiCl–KCl–MgCl2–MnCl2 melts at 893 K to form different phases Mg–Li–Mn alloys. Transient electrochemical techniques such as cyclic voltammetry, chronopotentiometry, and chronoamperometry have been used in order to investigate the codeposition behavior of Mg, Li, and Mn ions. The results obtained show that the potential of Li metal deposition, after the addition of MgCl2 and MnCl2, is more positive than the one of Li metal deposition before the addition. The codeposition of Mg, Li, and Mn occurs at current densities lower than −1.43 A cm−2 in LiCl–KCl–MgCl2 (8 wt%) melts containing 2 wt% MnCl2. The onset potential for the codeposition of Mg, Li, and Mn is −2.100 V. α, α + β, and β phases Mg–Li–Mn alloys with different lithium and manganese contents were obtained via galvanostatic electrolysis from LiCl–KCl melts with different concentrations of MgCl2 and MnCl2. The microstructures of typical α and β phases of Mg–Li–Mn alloys were characterized by X-ray diffraction (XRD), optical microscopy (OM), and scanning electron microscopy (SEM). The analysis of energy dispersive spectrometry (EDS) and EPMA area analysis showed that the elements of Mg and Mn distribute homogeneously in the Mg–Li–Mn alloys. The results of inductively coupled plasma analysis determined that the chemical compositions of Mg–Li–Mn alloys correspond with the phase structures of XRD patterns, and lithium and manganese contents of Mg–Li–Mn alloys depend on the concentrations of MgCl2 and MnCl2.









Similar content being viewed by others
References
Wang S, Wu G, Li R et al (2006) Mater Lett 60:1863
Drozd Z, Trojanová Z, Kúdela S (2004) J Alloys Compd 378:192
Sanschagrin A, Tremblay R, Angers R et al (1996) Mater Sci Eng A 220:69
Li H, Ji H, Yao G et al (2006) Chin J Process Eng 6:491 (in Chinese)
Li H (2009) Mater Heat Treat 38:12 (in Chinese)
Yan Y, Zhang M, Han W et al (2008) Electrochim Acta 53:3323
Zhang M, Yan Y, Hou Z et al (2007) J Alloys Compd 440:362
Castrillejo Y, Martínez AM, Pardo R et al (1997) Electrochim Acta 42:1869
Martínez AM, Børresen B, Haarberg GM et al (2004) J Electrochem Soc 151:C508
Martínez AM, Børesen B, Haarberg GM et al (2004) J Appl Electrochem 34:1271
Børresen B, Haarberg GM, Tunold R (1997) Electrochim Acta 42:1613
Carlin RT, Osteryoung RA (1989) J Electrochem Soc 136:1249
Piersma BJ, Ryan DM (1996) J Electrochem Soc 143:908
Tsuda T, Hussey CL, Stafford GR et al (2003) J Electrochem Soc 150:C234
Tsuda T, Hussey CL, Stafford GR (2004) J Electrochem Soc 151:C379
Tsuda T, Hussey CL, Stafford GR et al (2004) J Electrochem Soc 151:C447
Ueda M, Kigawa H, Ohtsuka T (2007) Electrochim Acta 52:2515
Yan Y, Zhang M, Han W et al (2008) Chem Lett 37:212
Yan Y, Zhang M, Xue Y et al (2009) Electrochim Acta 54:3387
Yan Y, Zhang M, Xue Y et al (2009) J Appl Electrochem 39:455
Tsuda T, Hussey CL, Stafford GR (2005) J Electrochem Soc 152:C620
Tsuda T, Arimoto S, Kuwabata S et al (2008) J Electrochem Soc 155:D256
Massalski TB, Murray JL, Benett LH et al (1990) Binary alloy phase diagrams. American Society for Metals, Metals Park
Wang D, Zhang J, Du H et al (2005) Res Stud Foundry Equip 1:12 (in Chinese)
Acknowledgments
This study was financially supported by the 863 project of the Ministry of Science and Technology of China (2006AA03Z510), the National Natural Science Foundation of China (50871033), the Scientific Technology Project of Heilongjiang Province (GC06A212), and the Scientific Technology Bureau of Harbin (2006PFXXG006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, K., Zhang, M.L., Chen, Y. et al. Study on the preparation of Mg–Li–Mn alloys by electrochemical codeposition from LiCl–KCl–MgCl2–MnCl2 molten salt. J Appl Electrochem 40, 1387–1393 (2010). https://doi.org/10.1007/s10800-010-0106-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-010-0106-x