Abstract
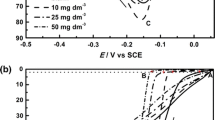
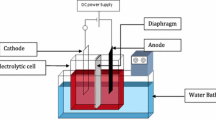
The effects of the organic additives 1-hexyl-3-methylimidazolium hydrogen sulfate ([HMIM]HSO4) and 1-octyl-3-methylimidazolium hydrogen sulfate ([OMIM]HSO4) on current efficiency (CE), power consumption (PC), polarization behavior of the cathode, deposit morphology, and crystallographic orientation during electrodeposition of zinc from acidic sulfate solution were investigated. The results were compared with those of a common industrial additive, gum arabic. Addition of these additives increases current efficiency, decreases power consumption, and improves the surface morphology at lower concentrations. Both the additives showed similar polarization behavior to gum arabic and the extent of polarization was in the order: gum arabic > [OMIM]HSO4 > [HMIM]HSO4. The nature of the electrode reactions was studied through measurements of Tafel slopes, transfer coefficients, and exchange current densities. Data obtained from X-ray diffractogram revealed that the presence of any of these additives did not change the structure of the electrodeposited zinc but affected the crystallographic orientation of the crystal planes.





Similar content being viewed by others
References
Ivanov I (2004) Hydrometallurgy 72:73
Mackinnon DJ, Brannen JM, Kerby RC (1979) J Appl Electrochem 9:55
Mackinnon DJ, Brannen JM, Kerby RC (1979) J Appl Electrochem 9:71
Ault AR, Frazer EJ (1988) J Appl Electrochem 18:583
Muresan L, Maurin G, Oniciu L, Gaga D (1996) Hydrometallurgy 43:345
Robinson DJ, O′Keefe TJ (1976) J Appl Electrochem 6:1
MacKinnon DJ, Brannen JM (1977) J Appl Electrochem 7:451
MacKinnon DJ, Brannen JM, Fenn PL (1987) J Appl Electrochem 17:1129
Das SC, Singh P, Hefter GT (1996) J Appl Electrochem 26:1245
Das SC, Singh P, Hefter GT (1997) J Appl Electrochem 27:738
Sato R (1959) J Electrochem Soc 106:206
Piron DL, Mathieu D, Amboise MD (1987) Can J Chem Eng 65:685
Hosny AY (1993) Hydrometallurgy 32:261
Karavasteva M, Karaivanov SA (1993) J Appl Electrochem 23:763
Karavasteva M (1994) Hydrometallurgy 35:391
Tripathy BC, Das SC, Singh P, Hefter GT (1997) J Appl Electrochem 27:673
Tripathy BC, Das SC, Singh P, Hefter GT (1999) J Appl Electrochem 29:1229
Tripathy BC, Das SC, Hefter GT, Singh P (1998) J Appl Electrochem 28:915
Tripathy BC, Das SC, Singh P, Hefter GT, Misra VN (2004) J Electroanal Chem 565:49
Forsyth SA, Pringle JM, MacFarlane DR (2004) Aust J Chem 57:113
Endres F, El Abedin SZ, Matter S (2006) Phys Chem Chem Phys 8:2101
Jiménez AE, Bermúdez MD, Iglesias P, Carrión FJ, Martínez-Nicolás G (2006) Wear 260:766
Kamimura H, Kubo T, Minami I, Mori S (2007) Tribo Int 40:620
Welton T (1999) Chem Rev 99:2071
Zhou Y (2005) Curr Nanosci 1:35
Xiao XH, Zhao L, Liu X, Jiang SX (2004) Anal Chim Acta 519:207
Zhang CD, Malhotra SV (2005) Talanta 67:560
Zhang QB, Hua YX (2008) Effects of 1-Butyl-3-Methylimidazolium Hydrogen Sulfate-[BMIM]HSO4 on zinc electrodeposition from acidic sulfate electrolyte. J Appl Electrochem. doi:10.1007/s10800-008-9665-5
Shi SC, Yi PG, Cao CZ, Wang XY (2005) J Chem Ind Eng China 56:1112
Huddleston JG, Visser AE, Reichert WM, Willauer HD, Broker GA, Rogers RD (2001) Green Chem 3:156
Whitehead JA, Lawrance GA, McCluskey A (2004) Aust J Chem 57:151
Stupnisek-Lisac E, Podbrscek S, Soric T (1994) J Appl Electrochem 24:779
Mohanty US, Tripathy BC, Singh P, Das SC (2001) J Appl Electrochem 31:969
Acknowledgements
The authors thank XinSheng Li for assistance in SEM and gratefully acknowledge the financial support of the National Natural Science Foundation of China (Project No. 50564006) and the Natural Science Foundation of Yunnan Province (Project No. 2005E0004Z).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Q., Hua, Y. Effects of [HMIM]HSO4 and [OMIM]HSO4 on the electrodeposition of zinc from sulfate electrolytes. J Appl Electrochem 39, 1185–1192 (2009). https://doi.org/10.1007/s10800-009-9783-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-009-9783-8