Abstract
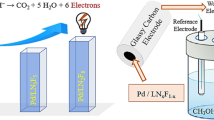
Metallic nanoaggregates deposited on non-conductive oxides powders as catalysts have shown good efficiency in electrocatalytic hydrogenation (ECH). In this process, the polarization of the metallic nanoaggregates is very important. This polarization can be improved when the electrode material is conductive. Thus, the goal of this work was to study the effect of the conduction of the supported material on the ECH process. Tin dioxide was chosen as oxide because it can be obtained in non-conductive or conductive form by doping with fluorine. Palladium supported catalysts powders were prepared by the sol–gel method. These electrocatalysts were characterized by XRD, SEM, TGA/DSC, FTIR and electrical conductivity. The effects of temperature and time of calcination were also investigated. Comparison of non-conductive and conductive catalysts for ECH of phenol shows that conductive F-doped SnO2 increases the rate of electrohydrogenation.















Similar content being viewed by others
References
Cheng IF, Fernando Q, Korte N (1997) Environ Sci Technol 31:1074
Ruest L, Ménard H, Moreau V, Laplante F (2002) Can J Chem 80:1662
Dubé P, Kerdouss F, Laplante F, Proulx P, Brossard L, Ménard H (2003) J Appl Electrochem 33:541
Mahdavi B, Lafrance A, Martel A, Lessard J, Ménard H (1997) J Appl Electrochem 27:605
Kung HH, Brookes BI, Burwell JRL (1974) J Phys Chem 78:875
Wismeijer AA, Kieboom APG, Van Bekkum H (1986) Recl Trav Chim Pays-Bas 105:129
Casadei MA, Pletcher D (1988) Electrochim Acta 33:117
Amouzegar K, Savadogo O (1997) J Appl Electrochem 27:539
Dabo P, Cyr A, Lessard J, Brossard L, Ménard H (1999) Can J Chem 77:1225
Laplante F, Bouchard NA, Dubé P, Ménard H, Brossard L (2003) Can J Chem 81:1039
Dubé P, Brossard L, Ménard H (2002) Can J Chem 80:345
Miller LL, Christensen L (1978) J Org Chem 43:2059
Misra RA, Sharma BL (1979) Electrochim Acta 24:727
Park C, Keane MA (2003) J Colloid Interface Sci 266:183
Cirtiu CM, Hassani HO, Bouchard NA, Rowntree PA, Ménard H (2006) Langmuir 22:6414
Liberkova K, Touroude R (2002) J Mol Catal A Chem 180:221
Rodrigues ECPE, Olivi P (2003) J Phys Chem Solids 64:1105
Siciliano P (2000) Sen Actuators B 70:153
Esteves MC, Gouvêa D, Sumodjo PTA (2004) Appl Surf Sci 229:24
Mahfouz RM, Alshhri SM, Monshi MAS, El-Salam NMAS (2004) Radiat Eff Defects Solid 159:345
Rella R, Serra A, Siciliano P, Vasanelli L, De G, Licciulli A (1997) Thin Solid Films 304:339
Zhu G, Han J, Zemlyanov DY, Ribeiro FH (2005) J Phys Chem B 109:2331
Zhang G, Liu M (1999) J Mat Sci 34:3213
Maxted EB, Ali SI (1961) J Chem Soc 83:4137
St-Pierre G, Chagnes A, Bouchard NA, Harvey PD, Brossard L, Ménard H (2004) Langmuir 20:6365
Acknowledgements
We thank the “Coopération France-Burkina Faso” for the BGF funding and the University of Sherbrooke for financial and material support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tountian, D., Brisach-Wittmeyer, A., Nkeng, P. et al. Effect of support conductivity of catalytic powder on electrocatalytic hydrogenation of phenol. J Appl Electrochem 39, 411–419 (2009). https://doi.org/10.1007/s10800-008-9686-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-008-9686-0