Abstract
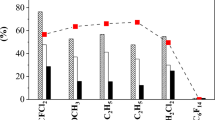
Electrochemical perfluorination (ECPF) of n-hexanoyl, n-heptanoyl, n-octanoyl, n-nonanoyl and n-decanoyl chlorides was carried out under identical experimental conditions in liquid HF. The product distribution among perfluorinated carboxylic acids, perfluoro ethers, perfluoroalkanes, isomerised and fragmented products containing less number of carbon atoms was identified using 19F NMR. The selectivity of C6–C10 perfluoro carboxylic acid varied between 29 and 36%. The alkali insoluble perfluoro cyclic ether and perfluoro alkane fractions increased with increasing chain length. The increase of perfluoroalkane fractions is mainly due to decarboxylation. Cyclic ether fractions also decreased slightly with increase in chain length. Among the cyclic ethers α substituted oxolanes were the predominant products. Six membered cyclic ethers were always found to contain β substitution. The possible pathways for these products are also indicated.





Similar content being viewed by others
References
Diesslin AR, Kauck EA (1951) Fluoro carbon acids and derivatives. US Patent no. 2567011
Kauck EA, Minn St.P, Simons JH (1952) Cyclic fluoro alkylene oxide compounds. US Patent no. 2594272
Kauck EA, Minn St.P, Simons JH (1953) Cyclic fluorocarbon ethers. US Patent no. 2644823 (1954) British Patent no. 718318
Ludwigshalle S A-G (1959) Method of producing organic cyclic Perfluoro ethers having 5 or 6-membered rings. German Patent no. 1069639 (1961) British Patent no. 862538
Abe T, Nagase S (1982) In: Banks RE (ed) Preparation properties and Industrial application of organofluorine compounds. Ellis Horwood, Chichester, pp 19–33
Childs WV, Christensen L, Klink FW, Kolpin CF (1991) In: Lund H, Baizer MM (eds) Organic electrochemistry. Marcel Dekker, New York, pp 1103–1127
Alsmeyer YW, Childs WV, Flynn RM, Moore GGI, Smeltzer JC (1994) In: Banks RE, Smart BE, Tatlow JC (eds) Organofluorine chemistry. Plenum Press, New York, pp 121–143
Drakesmith FG (1997) Electrofluorination of organic compounds. In: Chambers RD (ed) Organofluorine chemistry: techniques and synthons, topics in current chemistry, vol 193. Verlag, Berlin, pp 197–242
Sartori P, Ignatev N (1998) J Fluor Chem 87:157
Abe T (2000) J. Fluor Chem 105:181
Conte L, Gamberetto G (2004) J Fluor Chem 125:139
Abe T, Nagase S, Baba H (1976) Bull Chem Soc Jpn 49:1888
Abe T, Kodaira K, Baba H, Nagase S (1978) J Fluor Chem 12:1
Abe T, Nagase S (1979) J Fluor Chem 13:519
Comninellis Ch, Javet Ph, Plattner E (1974) J Appl Electrochem 4:289
Drakesmith FG, Hughes DA (1979) J Appl Electrochem 9:685
Lines D, Sutcliffe H (1981) J Fluor Chem 17:423
Drakesmith FG, Hughes DA (1986) J Fluor Chem 32:103
Prokop HW, Zhou H-J, Xu S-Q, Wu C-H, Chuey SR, Liu CC (1989) J Fluor Chem 43:257
Prokop HW, Zhou H-J, Xu S-Q, Wu C-H, Chuey SR, Liu CC (1989) J Fluor Chem 43:277
Napoli M, Conte L, Gambaretto GP (1989) J Fluor Chem 45:213
Chambers RD, Fuss RW, Jones M, Sartori P, Swales AP, Herkelmann R (1990) J Fluor Chem 49:409
Napoli M, Scipioni A, Gambaretto GP, Carlini F M, Bertola M (1994) J Fluor Chem 67:261
Scholberg HM, Brice HG (1955) US Patent no. 2717871
Gramstad T, Haszeldine RN (1956) J Chem Soc 173 (1957) J Chem Soc 2460
Brice TH, Trott PW (1956) US Patent no. 2732398
Murray J (1977) Unpublished work as cited by A. J. Rudge in: Industrial Electrochemical Process, A. T. Huhn (ed) Elsevier, Amsterdam pp 77
Kauck EA, Diesslin AR (1951) Ind Eng Chem 43:2332
Ignatev NV, Wek-Biermann U, Heider U, Kucheryma A, Ansen SV, Hubel W, Sartori P, Willner H (2003) J Fluor Chem 124:21
Velayutham D, Jayaraman K., Kulangiaappar K, Ilayaraja N, Rambabu Y, Santhan Rao P, Narayana Reddy S, Victor Babu K, Noel M (2006) J Fluor Chem 127:1111
Acknowledgements
The authors thank the Ministry of Environment & Forests, Govt. of India, for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10800-007-9443-9
Rights and permissions
About this article
Cite this article
Ilayaraja, N., Manivel, A., Velayutham, D. et al. Effect of alkyl chain length on the electrochemical perfluorination of n-alkane (C6–C10) carboxylic acid chlorides. J Appl Electrochem 38, 175–186 (2008). https://doi.org/10.1007/s10800-007-9421-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-007-9421-2