Abstract
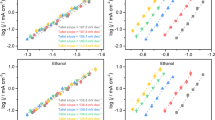
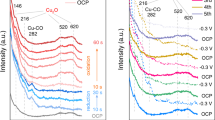
The electrocatalytic behaviour of copper in acid is dominated by a metastable state interfacial redox transition which occurs within the double layer region, at ca. −0.7 (±0.1) V (SMSE). The transition in question is apparently based on the presence of low coverage, redox active, copper surface clusters which function as active sites or mediators and thus facilitate electron transfer across the interface; copper in acid exhibits a remarkably high overpotential of ca. 1.2 V for oxygen reduction. The effect of copper plating bath additives on the electrocatalytic properties of copper was surveyed and it was demonstrated that with a compound such as thiourea, the presence in solution of a species such as nitrate, which under additive-free conditions undergoes rapid mediated reduction, exacerbates the surface deactivating effect of the additive.









Similar content being viewed by others
References
Andricacos PC (1999) The Electrochem Soc 8:32
Andricacos PC, Uzoh C, Dukovic JO, Horkans J, Deligianni H (1998) IBM J Res Dev 42:567
Hussein MA, He J (2005) IEEE Trans Semiconductor Manuf 18:69
Kang M, Gross ME, Gerwirth AA (2003) J Electrochem Soc 150:C292
Moffat TP, Wheeler D, Edelstein ED, Josell D (2005) IBM J Res Dev 49:19
Pletcher D, Poorabedi Z (1979) Electrochim Acta 24:1253
Albery WJ, Haggett BGD, Jones CP, Prichard MJ, Svanberg LR (1985) J Electroanal Chem 188:257
Dima GE, de Vooys ACA, Koper MTM (2003) J Electroanal Chem 554:15
Marquez J, Pletcher D (1980) J Appl Electrochem 10:567
Nolen TR, Fedkiw PS (1990) J Appl Electrochem 20:370
Polat K, Aksu ML, Pekel AT (2002) J Appl Electrochem 32:217
Nolen TR (1988) J Electrochem Soc Rev News 135:29C
Burke LD, O’Connell AM, Sharna R, Buckley CA (2006) J Appl Electrochem 36:919
Burke LD, Buckley CA, Sharna R (2007) ESC Trans 2(6):197
Pourbaix M (1966) Atlas of electrochemical equilibria in aqueous solutions. Pergammon Press, Oxford
Andrews LJ, Keefer RM (1950) J Am Chem Soc 72:3113
Hori Y, Murata A, Takahashi R (1989) J Chem Soc Faraday Trans 85:2309
Das TN (2005) Ind Eng Chem Res 44:1660
EI-Deab MS, Okajima T, Ohsaka T (2003) J Electrochem Soc 150:A851
Vukmirovic MB, Vasiljevic N, Dimitrov N, Sieradzki K (2003) J Electrochem Soc 150:B10
Metikoš-Hukovic’ M, Babic’ R, Jovic’ F, Grubač Z (2006) Electrochim Acta 51:1157
Sarapuu A, Tammeveski K, Tenno TT, Sammelselg V, Kontturi K, Schiffrin DJ (2001) Electrochem Commun 3:446
Ertl G (2000) In: Gates BC, Knözinger H (eds) Advances in catalysis, vol 45. Academic, New York
Somorjai GA (1977) In: Eley DD, Pines H, Weisz PB (eds) Advances in catalysis, vol 26. Academic, New York
Dieluweit S, Giesen M (2002) J Electroanal Chem 524:194
Burke LD (2004) Gold Bull 37:125
Burke LD, Collins JA, Murphy MA (1999) J Solid State Electrochem 4:34
Pletcher D (1984) J Appl Electrochem 14:403
Burke LD, Nugent PF (1999) Electrochim Acta 42:399
Hernández J, Solla-Gullón J, Herrero E, Aldaz A, Feliu JM (2006) Electrochim Acta 52:1662
Yokoi M, Konishi S, Hayashi T (1983) Denki Kagaku 51:460
Nagy Z, Blaudeau JP, Hung NC, Curtiss LA, Zurawski DJ (1995) J Electrochem Soc 142:L87
Nakahara S, Ahmed S, Buckley DN (2007) Electrochem Solid-State Lett 10:D17
Healy JP, Pletcher D, Goodenough M (1992) J Electroanal Chem 338:155
Healy JP, Pletcher D (1992) J Electroanal Chem 338:179
Vereecken PM, Binsread RA, Deligianni H, Andricacos PC (2005) IBM J Res Dev 49:3
Taephaisitphongse P, Cao Y, West AC (2001) J Electrochem Soc 148:C492
Murray RW (1984) In: Bard AJ (ed) Electroanalytical chemistry, vol 13. Dekker, New York
Taniguchi I (1997) The Electrochem Soc Interface 6:34
Acknowledgements
This material is based on work supported by Science Foundation Ireland (SFI) under Grant No. 02/INI/1217; RS was awarded an SFI postgraduate research studentship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burke, L.D., Sharna, R. Surface active state involvement in electrocatalytic reductions at copper in acid solution. J Appl Electrochem 37, 1119–1128 (2007). https://doi.org/10.1007/s10800-007-9370-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-007-9370-9