Abstract
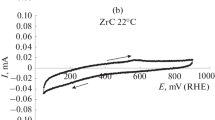
Thermodynamic predictions are reported for platinum and palladium in aqueous ammonia and iodide solutions, to define less aggressive conditions than used hitherto for leaching palladium and platinum from secondary materials. Cyclic voltammetry and amperometry of thin films of palladium, electrodeposited onto rotating vitreous carbon disc electrodes, indicated that partially oxidised adsorbed species passivated dissolution in aqueous ammonium sulfate. By contrast, dissolution rates in aqueous potassium iodide solutions were a significant fraction of that corresponding to the mass transport controlled rate of reduction of tri-iodide, which was demonstrated to be a suitable oxidant for the envisaged metal recovery process. However, iodide concentrations > 1 m were required to achieve adequate solubility of the oxidation products, assumed to be PdI 2−4 ions, thereby avoiding inhibition by PdI2. The reduction of tri-iodide on palladium was very facile, with large exchange current densities and Tafel coefficients; two alternative mechanisms are proposed that fitted experimental results well. In addition, a kinetic model to predict dissolution rates of Pd in tri-iodide solutions gave good agreement with experimental data, provided an equilibrium constant of 10−4.5 was used for the PdI2/PdI 2−4 reaction, rather than the value of 10−2.8 derived from thermodynamic data.














Similar content being viewed by others
Abbreviations
- d :
-
effective thickness of PdI2 (c) layer, m
- D :
-
diffusion coefficient, m2 s−1
- f :
-
rotation rate of (ring-)disc electrode, Hz
- F :
-
Faraday constant, 96 485, C mol−1
- I d :
-
disc electrode current, A
- I r :
-
ring electrode current, A
- j :
-
current density, A m−2
- j 0 :
-
exchange current density, A m−2
- j L :
-
mass transport limited current density, A m−2
- k m :
-
mass transport rate coefficient, m s−1
- k i :
-
standard rate coefficient for reaction i, m s−1
- K :
-
equilibrium constant, mol−1 dm3
- M PdI 2 :
-
molar mass of PdI2 (c), 360.23, g mol−1
- n :
-
electron stoichiometric factor/reaction charge number, 1
- n d :
-
electron stoichiometric factor/reaction charge number for disc electrode, 1
- n r :
-
electron stoichiometric factor/reaction charge number for ring electrode, 1
- N 0 :
-
collection efficiency, 1
- r 1 :
-
disc electrode radius, 2.285, mm
- r 2 :
-
ring electrode inner radius, 4.93, mm
- r 3 :
-
ring outer radius, 5.38, mm
- α:
-
transfer coefficient, 1
- β:
-
Tafel coefficient for reduction reaction, V−1
- β′:
-
Tafel coefficient for oxidation reaction, V−1
- δ:
-
diffusion layer thickness, m
- η:
-
overpotential (E − E r), V
- ν:
-
kinematic viscosity of 4 m KI electrolyte solution at 298 K, 0.6 × 10−6 (0.72 × 10−6 for 2 m, 0.962 × 10−6 for 0.2 m), m2 s−1
- θ:
-
fractional coverage, 1
- ρ PdI 2 :
-
density of PdI2 (c), 6.003, g cm−3
- Ψ:
-
conversion factor in equations 16, 18, 19, 21, m
References
R.K. Mishra, Precious Metals ’89. The Metallurgical Society (Warrendale, PA, USA, 1989) 483 pp
J.E. Hoffmann, Precious and Rare Metal Technologies. (Elsevier, BV, 1988) 345 pp
V.I. Lakshmanan and J. Ryder, (1988) Precious and Rare Metal Technologies (Elsevier, BV, J.) 381 pp
J.A. Bonucci and P.D. Parker, Precious Metals Mining, Extraction and Processing (The Metallurgical Society, Warrendale, PA, USA, 1984), 27 pp
T.N. Angelidis, Top. Catal. 16 (2001) 419
X. Meng and K. Han, Miner. Metall. Process. 15 (1998) 36
X. Meng and K. Han, EPD Congress 1996. (The Minerals, Metals and Materials Society, Warrendale, PA, USA, 1996) 36 pp
X. Meng and K. Han, Third International Symposium, Recycling of Metals and Engineered Materials (The Minerals, Metals and Materials Society, Warrendale, PA, USA, 1995) 501 pp
W. Albery and M. Hitchman, Ring-Disc Electrodes (Clarendon Press, Oxford University Press, UK, 1971) 22 pp
Choi J.C., Lee W.H. (1995). Han’guk Chawon Konghak Hoechi. 32:357
J. Mansikka-aho and A. Roine, HSC Chemistry 5.11, Outokumpu Research, http://www.outokumputechnology.com, (2003)
A.J. Bard, R. Parsons and J. Jordan, Standard Potentials in Aqueous Solution (Dekker, New York, USA, 1983) 321 pp
Goldberg R.N., Hepler L.G. (1968). Chem. Rev. (Washington, DC, U.S.) 68:229
Kelsall G.H., Welham N.J., Diaz M.A. (1993) J. Electroanal. Chem. 36:13
Bard A.J. (1985). Iodine and Astatine. In: Bard A.J., Stratmann M. (eds) Encyclopaedia of Electrochemistry. Vol.B2, Wiley, Weinheim, DE, pp. 104–145
de Mishima B.A.L., Lescano D., Holgado T.M., Mishima H.T. (1998). Electrochim. Acta 43:395
de Vooys A.C.A., Koper M.T.M., van Santen R.A., van Veen J.A.R. (2001). J Electroanal Chem 506:127
Vidal-Iglesias F.J., Garcia-Araez N., Montiel V., Feliu J.M., Aldaz A. (2003). Electrochem. Commun. 5:22
D.R. Tyson and R.G. Bautista, Sep. Sci. Technol. (1987) 1149
Dane L.M., Janssen L.J.J., Hoogland J.G. (1968). Electrochim. Acta 13:507
Macagno V.A., Giordano M.C., Arvia A.J. (1969). Electrochim. Acta 14:335
Hauch A., Georg A. (2001). Electrochim. Acta 46:3457
Bay L., West K., Winther-Jensen B., Jacobsen T.(2006). Sol. Energy Mater. Sol. Cells 90:341
Acknowledgements
The authors thank the UK Engineering and Physical Sciences Research Council and the Resource Efficiency Knowledge Transfer Network for a studentship for RJD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dawson, R., Kelsall, G. Recovery of platinum group metals from secondary materials. I. Palladium dissolution in iodide solutions. J Appl Electrochem 37, 3–14 (2007). https://doi.org/10.1007/s10800-006-9218-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-006-9218-8