Abstract
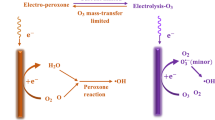
An electrochemical reactor for oxygen/ozone production was developed using perforated planar electrodes. An electroformed \(\beta\)-PbO2 coating, deposited on a platinised titanium substrate, was employed as anode while the cathode was a platinised titanium substrate. The electrodes were pressed against a solid polymer electrolyte to minimise ohmic drop and avoid mixing of the gaseous products (H2 and O2/O3). Electrochemical ozone production (EOP) was investigated as function of current density, temperature and electrolyte composition. Electrochemical characterisation demonstrated ozone current efficiency, ΦEOP, ozone production rate (g h−1), \(\nu_{\rm EOP}\), and grams of O3 per total energy demand (g h−1 W−1), \(\nu_{\rm EOP}\) increase on decreasing electrolyte temperature and increasing current density. The best reactor performance for EOP was obtained with the base electrolyte (H2SO4 3.0 mol dm−3) containing 0.03 mol dm−3 KPF6. Degradation of reactive dyes used in the textile industry (Reactive Yellow 143 and Reactive Blue 264) with electrochemically-generated ozone was investigated in alkaline medium as function of ozone load (mg h−1) and ozonation time. This investigation revealed ozonation presents very good efficiency for both solution decolouration and total organic carbon (TOC) removal.
Similar content being viewed by others
References
Jüttner H., Galla U. and Schmieder H. (2000) Electrochim. Acta 45:2575
Da Silva L.M., De Faria L.A. and Boodts J.F.C. (2001) Pure Appl. Chem. 73:1871
Da Silva L.M., Santana M.H.P. and Boodts J.F.C. (2003) Quim. Nova 26:880
Walsh F.C. (2001) Pure Appl. Chem. 73:1819
Rice R.G. and Netzer A. (1982) Handbook of Ozone Technology and Applications. Ann Arbor Science, New York
Tatapudi P. and Fenton J.M. (1994) In: Sequeira C.A.C., (eds), Environmental Oriented Electrochemistry. Elsevier, Amsterdam, The Netherlands, pp. 103
Winarno E.K. and Getoff N. (2002) Radiat. Phys. Chem. 65:387
Stucki S., Theis G., Kötz R., Devantay H. and Christen H.J. (1985) J. Electrochem. Soc. 132:367
Stucki S., Baumann H., Christen H.J. and Kötz R. (1987) J. Appl. Electrochem. 17:773
Zhou Y., Wu B., Gao R., Zhang H. and Jiang W. (1996) Yingyoung Huaxue 13:95
Graves J.E., Pletcher D., Clarke R.L. and Walsh F.C. (1992) J. Appl. Electrochem. 22:200
Foller P.C. and Kelsall G.H. (1993) J. Appl. Electrochem. 23:996
Simond O. and Comninellis Ch. (1997) Electrochim. Acta 42:2013
Da Silva L.M., de Faria L.A. and Boodts J.F.C. (2003) Electrochim. Acta 48:699
O. Leitzke, Internationales Symposium Ozon und Wasser, Berlin, Germany, 14–17 September (1977) pp. 164–166
L.M. Da Silva, Investigation of the Electrochemical Technology for Ozone Production: Fundamentals and Applied Aspects, Thesis, Chemistry Department/FFCLRP, University of São Paulo, Ribeirão Preto (2004)
Foller P.C. and Tobias C.W. (1982) J. Electrochem. Soc. 129:505
Gellings P.J. and Bouwmeester H.J.M. (1996) The CRC Handbook of Solid State Electrochemistry. CRC Press, Enschede, The Netherlands
Scott K. (1995) Electrochemical Processes for Clean Technology. The Royal Society of Chemistry, Cambridge, UK
Selcuk H. (2005) Dyes Pigments 64:217
Chu W., Ma C.W. (2000) Wat. Res. 34:3153
Pontius F.W. (1990) Water Quality and Treatment. American Water Works Association, McGraw-Hill, New York
Zoubov N. and Pourbaix M. (1974) In: Pourbaix M., (eds), Atlas of Electrochemical Equilibria in Aqueous Solutions. NACE International, Texas, pp. 540
L.M. Da Silva and W.F. Jardim, Quim. Nova, in press.
Hao O.J., Kim H. and Pen-Chi Chiang (2000) Crit. Rev. Environm. Sci. Technol. 30:449
Kusvuran E., Gulnaz O., Irmak S., Atanur O.M., Yavuz H.I. and Erbatur O. (2004) J. Hazardous Mater. B109:85
Ince N.H. and Tezcanlí G. (2001) Dyes Pigments 49:145
Hsu Y.C., Chen J.T., Yang H.C. and Chen J.H. (2001) AIChE J. 47:169
Lin S.H. and Wang C.H. (2003) J. Hazardous Mater. B98:295
Acknowledgements
L.M. Da Silva, J.C.Fórti and W.F. Jardim acknowledge the FAPESP Foundation. D.V. Franco and J.F.C. Boodts thank the FAPEMIG Foundation. The authors also thank Dr. C. Collins.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Da Silva, L.M., Franco, D.V., Forti, J.C. et al. Characterisation of a laboratory electrochemical ozonation system and its application in advanced oxidation processes. J Appl Electrochem 36, 523–530 (2006). https://doi.org/10.1007/s10800-005-9067-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-005-9067-x