Abstract
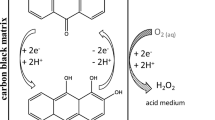
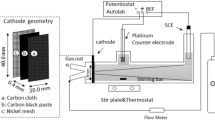
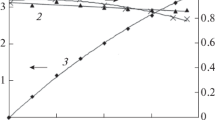
This work studies the production of hydrogen peroxide through the cathodic reduction of oxygen in acidic medium, by comparing the results obtained using a commercial graphite and a gas diffusion electrode. A low pH was required to allow the application of hydrogen peroxide generation to an electro-Fenton process. The influence of applied potential and the gas flow composition were investigated. The gas diffusion electrode demonstrates a higher selectivity for hydrogen peroxide production, without significantly compromising the iron regeneration, thus making its successful application to a cathodic Fenton-like treatment, possible. Unlike the graphite cathode, the gas diffusion cathode also proved to be effective in the air flow.
Similar content being viewed by others
References
D. Pletcher, Electrogenerated Hydrogen Peroxide-From history to new opportunities January 1999 Vol.4 No 1. http://www.electrosynthesis.com
B.C. Larish S.J.B. Duff (1997) Water Res 31 IssueID7 1694
L. Plant M. Jeff (1994) Chem. Eng. 9 EE16
P. Drogui S. Elmaleh M. Rumeau C. Bernard A. Rambaud (2001) J. Appl. Electrochem 31 877
M. Doré (1989) Chimie des oxydants et traitement des eaux Lavoisier Paris 250
A. Gallegos D. Pletcher (1998) Electrochimica Acta 44 853
J. De Laat H. Gallard S. Ancelin B. Legube (1999) Chemosphere 39 IssueID15 2693
H.J.H. Fenton (1894) J. Chem. Soc 65 899
F. Haber J. Weiss (1934) Proc. Royal Soc A 147 322
S.M. Arnold W.J. Hickey R.F. Harris (1995) Environ. Sci. Technol 29 2083
Y.W. Kang K.Y. Hwang (2000) Water Res 34 IssueID10 2786
A. Goi M. Trapido (2002) Chemosphere 46 913
E. Chamarro A. Marco S. Esplugas (2001) Water Res 35 IssueID4 1047
M.V. Balarama Krishna K. Chandrasekaran D. Karunasagar J. Arunachalam (2001) J. Haz. Mat. B 84 229
J.S. Do W.C. Yeh (1998) J. Appl. Electrochem 28 703
M.A. Oturan (2000) J. Appl. Electrochem 30 475
T. Tzedakis A. Savall M.J. Clifton (1989) J. Appl. Electrochem. J. Phys. Chem 19 911
A. Alvarez-Gallegos D. Pletcher (1999) Electrochimica Acta 44 2483
S. Chou Y.H. Huang S.N. Lee G.H. Huang C. Huang (1999) Wat. Res 33 IssueID3 751
Y.L. Hsiao K. Nobe (1993) J. Appl. Electrochem 23 943
M.A. Oturan N. Oturan C. Lahitte S. Trevin (2001) J. Electroanal. Chem 50 796
M. Panizza G. Cerisola (2001) Water Res 35 IssueID16 3987
C. Ponce De Leon D. Pletcher (1995) J. Appl. Electrochem 25 307
K. Pratap A.T. Lemley (1998) J. Agric. Food Chem 46 3285
A. Ventura A. Jacquet A. Bermond V. Camel (2002) Water Res 36 3517
M. Sudoh M. Kitaguchi K. Koide (1985) J. Chem. Eng. Jpn 18 IssueID5 409
E.E. Kalu C. Oloman (1990) J. Appl. Electrochem 20 932
P.C. Foller R.T. Bombard (1995) J. Appl. Electrochem 25 613
E. Brillas R.M. Bastida E. Llosa J. Casado (1995) J. Electrochem. Soc 142 IssueID6 1733
E. Brillas E. Mur J. Casado (1996) J. Electrochem. Soc 143 L49
F. Alcaide E. Brillas P.L. Cabot (2002) J. Electrochem. Soc 149 IssueID2 E64
T. Harrington D. Pletcher (1999) J. Electrochem. Soc 146 IssueID8 2983
Z. Qiang J.H. Chang C.P. Huang (2002) Water Res 36 85
APHA, AWWA, WPCF, Standard Method for the Examination of Water and Wastewater (Baltimore, MA, 1989) 19th ed
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pozzo, A., Palma, L., Merli, C. et al. An experimental comparison of a graphite electrode and a gas diffusion electrode for the cathodic production of hydrogen peroxide. J Appl Electrochem 35, 413–419 (2005). https://doi.org/10.1007/s10800-005-0800-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10800-005-0800-2