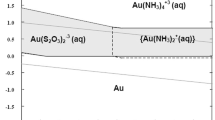
Abstract
Thiosulfate has been considered as one of the most promising of the non-toxic alternatives to cyanide for the leaching of gold and much work has been carried out with the aim of understanding and improving the ammoniacal thiosulfate leaching process. In particular the behaviour of gold in thiosulfate solutions containing copper in the absence of ammonia has received little attention. It has been shown in this study involving electrochemical and leaching tests that copper ions catalyze not only the oxidation of thiosulfate but also the dissolution of gold in alkaline thiosulfate solutions. Electrochemical studies have shown that copper has a positive effect on the anodic dissolution of gold with increasing concentrations of copper resulting in higher dissolution rates of gold at a potential of 0.3 V. Studies on the dissolution of gold powder in alkaline oxygenated thiosulfate solutions containing low concentrations of copper have shown that the role of copper in enhancing the dissolution rate of gold is possibly associated with the formation of a copper–thiosulfate–oxygen intermediate which is more reactive in terms of cathodic reduction than dissolved oxygen. The electrochemical experiments have been complemented by a leaching study which has shown that milling of gold powder in the presence of copper (added as ions, metal, or oxide) assists with the dissolution of gold in thiosulfate solutions.
Similar content being viewed by others
References
M.G. Aylmore D.M. Muir (2001) Minerals Eng. 14 135
R.Y. Wan, in World Gold ‘97 Conference, Singapore, (Australasian Institute of Mining and Metallurgy, 1997), p. 159.
H.A. White (1905) J. Chem. Metall. Min. Soc. S. Afr. 5 109
J.G. Webster (1986) Geochim. Cosmochim. Acta 50 1837
S. Zhang M.J. Nicol (2003) J. Appl. Electrochem. 33 767
C. Abbruzzese P. Fornari R. Massidda F. Veglio S. Ubaldini (1995) Hydrometallurgy 39 265
D. Zipperian S. Raghavan J.P. Wilson (1988) Hydrometallurgy 19 361
P.L. Breuer M.I. Jeffrey (2000) Minerals Eng. 13 1071
N.G. Tyurin I.A. Kakovskii (1960) Izvest. Vysshikh Ucheb. Zavedenii Tsvetnaya Met. 3 6
K.A. Ter-Arakelyan K.A. Bagdasaryan A.G. Oganyan R.T. Mkrtchyan G.G. Babayan (1984) Izv. Vyssh. Uchebn. Zaved Tsvetn. Metal. 5 72
P.L. Breuer M.I. Jeffrey (2002) Hydrometallurgy 65 145
P.L. Breuer M.I. Jeffrey (2003) Minerals Eng. 16 21
J.J. Byerley S.A. Fouda G.L. Rempel (1973) J. Chem. Soc. Dalton Trans. 8 889
J.J. Byerley S.A. Fouda G.L. Rempel (1975) J. Chem. Soc. Dalton Trans. 13 1329
J.W. Mellor (1929) A comprehensive treatise on inorganic and theoretical chemistry, Vol. X Longmans London 530–531
D.S. Flett R. Derry J.C. Wilson (1983) Trans. Inst. Min. Metall Section C 92 216
P.L. Breuer, M.I. Jeffrey and W.L. Choo, in C. Young, L. Twidwell and C. Anderson(Eds), ‘Cyanide: Social, Industrial and Economic Aspects’, (TMS, Warrendale, Pennsylvania, 2001), p. 455.
G. Rabai I.R. Epstein (1992) Inorg. Chem. 31 3229
R. Briones G.T. Lapidus (1998) Hydrometallurgy 50 243
E. Rolia C.L. Chakrabarti (1982) Environ. Sci. Techno. 16 852
F.R. Hopf M.M. Rogic J.F. Wolf (1983) J. Phys. Chem. 87 4681
I. Pecht M. Anbar (1968) J. Chem. Soc. A. 8 1902
H. Nord (1955) Acta Chem. Scand. 9 430
A.D. Zuberbühler (1967) Helv. Chim. Acta 50 466
A.D. Zuberbühler (1983) NoChapterTitle J. Zubieta (Eds) Copper coordination chemistry: Biochemical and inorganic prospectives Academic Press New York 237
L.I. Simandi (1992) Catalytic activation of dioxygen by metal complexes, Vol. 13 Kluwer Academic Publishers Dordrecht, The Netherlands 396
M.I. Jeffrey (2001) Hydrometallurgy 60 7
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, S., Nicol, M.J. An electrochemical study of the dissolution of gold in thiosulfate solutions. Part II. Effect of Copper. J Appl Electrochem 35, 339–345 (2005). https://doi.org/10.1007/s10800-004-7469-9
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10800-004-7469-9