Abstract
Purpose
The aim of the present study was to investigate increase in delivery of drug upon formulation as mucoadhesive microemulsion system and further to investigate possibility of any cytotoxic effects using such formulation.
Material and methods
Considering hydrophilic and small molecular nature of the drug, it was attempted to be formulated as microemulsion, by using pseudo ternary phase diagram method. Thus, three types of microemulsions were prepared; oil in water, water in oil type and chitosan-coated microemulsion. These microemulsions were characterized for several physicochemical properties like size, zeta potential, Polydispersity index, and compared for in vitro cell viability and ex vivo corneal irritation study.
Results
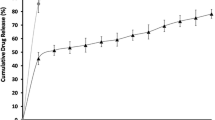
All three microemulsions were quite stable, transparent and homogenous systems. They showed similar drug release pattern, but highest ex vivo goat corneal permeation was observed with Chitosan coated microemulsion when compared with ganciclovir solution.
Conclusion
All microemulsions were found to be non-irritant in in vitro cell viability assay and ex vivo corneal irritation study, indicating the potential of using such systems for delivery of drug to eye.








Similar content being viewed by others
References
Nayak K, Misra M (2018) A review on recent drug delivery systems for posterior segment of eye. Biomed Pharmacother 107:1564–1582
Joseph RR, Venkatraman SS (2017) Drug delivery to the eye: What benefits do nanocarriers offer? Nanomedicine 12:683–702. https://doi.org/10.2217/nnm-2016-0379
Sánchez-López E, Espina M, Doktorovova S et al (2017) Lipid nanoparticles (SLN, NLC): overcoming the anatomical and physiological barriers of the eye—Part I—barriers and determining factors in ocular delivery. Eur J Pharm Biopharm 110:70–75. https://doi.org/10.1016/j.ejpb.2016.10.009
Addo RT (2016) Ocular drug delivery: advances, challenges and applications. Ocular drug delivery: advances, Challenges and Applications. R. T. Addo. Springer International Publishing, Berlin, pp 1–185
Barar J, Aghanejad A, Fathi M, Omidi Y (2016) Advanced drug delivery and targeting technologies for the ocular diseases. BioImpacts 6:49–67. https://doi.org/10.15171/bi.2016.07
Port AD, Orlin A, Kiss S et al (2017) Cytomegalovirus retinitis: a review. J Ocul Pharmacol Ther 33:224–234. https://doi.org/10.1089/jop.2016.0140
Lin CZ, Xiang GL, Zhu XH et al (2018) Advances in the mechanisms of action of cancer-targeting oncolytic viruses (review). Oncol Lett 15:4053–4060. https://doi.org/10.3892/ol.2018.7829
Yellepeddi VK, Palakurthi S (2016) Recent advances in topical ocular drug delivery. J Ocul Pharmacol Ther 32:67–82. https://doi.org/10.1089/jop.2015.0047
Al-Badr AA, Ajarim TDS (2018) Ganciclovir. In: Profiles of drug substances, excipients and related methodol. pp 1–208
Tirucherai GS, Dias C, Mitra AK (2002) Corneal permeation of ganciclovir: Mechanism of ganciclovir permeation enhancement by acyl ester prodrug design. J Ocul Pharmacol Ther 18:535–548. https://doi.org/10.1089/108076802321021081
Patel K, Trivedi S, Luo S et al (2005) Synthesis, physicochemical properties and antiviral activities of ester prodrugs of ganciclovir. Int J Pharm 305:75–89. https://doi.org/10.1016/j.ijpharm.2005.08.024
Majumdar S, Nashed YE, Patel K et al (2005) Dipeptide monoester ganciclovir prodrugs for treating HSV-1-induced corneal epithelial and stromal keratitis: In vitro and in vivo evaluations. J Ocul Pharmacol Ther 21:463–474. https://doi.org/10.1089/jop.2005.21.463
Shen Y, Tu J (2007) Preparation and ocular pharmacokinetics of ganciclovir liposomes. AAPS J 9:E371–E377. https://doi.org/10.1208/aapsj0903044
Merodio M, Irache JM, Valamanesh F, Mirshahi M (2002) Ocular disposition and tolerance of ganciclovir-loaded albumin nanoparticles after intravitreal injection in rats. Biomaterials 23:1587–1594. https://doi.org/10.1016/S0142-9612(01)00284-8
Herrero-Vanrell R, Ramirez L (2000) Biodegradable PLGA microspheres loaded with ganciclovir for intraocular administration. Enapsulation technique in vitro release profiles, and sterilization process. Pharm Res 17:1323–1328. https://doi.org/10.1023/A:1026464124412
Duvvuri S, Janoria KG, Pal D, Mitra AK (2007) Controlled delivery of ganciclovir to the retina with drug-loaded poly(D, L-lactide-co-glycolide) (PLGA) microspheres dispersed in PLGA-PEG-PLGA gel: A novel intravitreal delivery system for the treatment of cytomegalovirus retinitis. J Ocul Pharmacol Ther 23:264–274. https://doi.org/10.1089/jop.2006.132
Qaddoumi MG, Ueda H, Yang J et al (2004) The characteristics and mechanisms of uptake of PLGA nanoparticles in rabbit conjunctival epithelial cell layers. Pharm Res 21:641–648. https://doi.org/10.1023/B:PHAM.0000022411.47059.76
Akhter S, Ramazani F, Ahmad MZ et al (2013) Ocular pharmacoscintigraphic and aqueous humoral drug availability of ganciclovir-loaded mucoadhesive nanoparticles in rabbits. Eur J Nanomedicine 5:159–167. https://doi.org/10.1515/ejnm-2013-0012
Wang Q, Sun C, Xu B et al (2018) Synthesis, physicochemical properties and ocular pharmacokinetics of thermosensitive in situ hydrogels for ganciclovir in cytomegalovirus retinitis treatment. Drug Deliv 25:59–69. https://doi.org/10.1080/10717544.2017.1413448
Vandamme TF (2002) Microemulsions as ocular drug delivery systems: Recent developments and future challenges. Prog Retin Eye Res 21:15–34. https://doi.org/10.1016/S1350-9462(01)00017-9
Bachu RD, Stepanski M, Alzhrani RM et al (2018) Development and evaluation of a novel microemulsion of dexamethasone and tobramycin for topical ocular administration. J Ocul Pharmacol Ther 34:312–324. https://doi.org/10.1089/jop.2017.0082
Alany RG, Rades T, Nicoll J et al (2006) W/O microemulsions for ocular delivery: evaluation of ocular irritation and precorneal retention. J Control Release 111:145–152. https://doi.org/10.1016/j.jconrel.2005.11.020
Elbahwy IA, Lupo N, Ibrahim HM et al (2018) Mucoadhesive self-emulsifying delivery systems for ocular administration of econazole. Int J Pharm 541:72–80. https://doi.org/10.1016/j.ijpharm.2018.02.019
Ding D, Kundukad B, Somasundar A et al (2018) Design of mucoadhesive PLGA microparticles for ocular drug delivery. ACS Appl Bio Mater 1:561–571. https://doi.org/10.1021/acsabm.8b00041
Manchanda S, Sahoo PK (2018) Fabrication and characterization of mucoadhesive topical nanoformulations of dorzolamide HCl for ocular hypertension. J Pharm Investig 48:323–332. https://doi.org/10.1007/s40005-017-0324-x
Irimia T, Dinu-Pîrvu CE, Ghica MV et al (2018) Chitosan-based in situ gels for ocular delivery of therapeutics: a state-of-the-art review. Mar Drugs 16:1–23. https://doi.org/10.3390/md16100373
Alhakamy NA, Hosny KM (2019) Nano-vesicular delivery system loaded by bifonazole: preparation, optimization, and assessment of pharmacokinetic and antifungal activity. J Drug Deliv Sci Technol 49:316–322. https://doi.org/10.1016/j.jddst.2018.11.020
Alayoubi A, Aqueel MS, Cruz CN et al (2018) Application of in vitro lipolysis for the development of oral self-emulsified delivery system of nimodipine. Int J Pharm 553:441–453. https://doi.org/10.1016/j.ijpharm.2018.10.066
Padula C, Telò I, Di Ianni A et al (2018) Microemulsion containing triamcinolone acetonide for buccal administration. Eur J Pharm Sci 115:233–239. https://doi.org/10.1016/j.ejps.2018.01.031
Shah BM, Misra M, Shishoo CJ, Padh H (2014) Nose to brain microemulsion-based drug delivery system of rivastigmine: formulation and ex-vivo characterization. Drug Deliv 7544:1–13. https://doi.org/10.3109/10717544.2013.878857
Resende AP, Silva B, Braz BS et al (2017) Ex vivo permeation of erythropoietin through porcine conjunctiva, cornea, and sclera. Drug Deliv Transl Res 7:625–631. https://doi.org/10.1007/s13346-017-0399-y
Liu S, Dozois MD, Chang CN et al (2016) Prolonged ocular retention of mucoadhesive nanoparticle eye drop formulation enables treatment of eye diseases using significantly reduced dosage. Mol Pharm 13:2897–2905. https://doi.org/10.1021/acs.molpharmaceut.6b00445
Gagliano C, Papa V, Amato R et al (2018) Measurement of the retention time of different ophthalmic formulations with ultrahigh-resolution optical coherence tomography. Curr Eye Res 43:499–502
Teshima D, Otsubo K, Yoshida T et al (2003) A simple and simultaneous determination of acyclovir and ganciclovir in human plasma by high-performance liquid chromatography. Biomed Chromatogr 17:500–503. https://doi.org/10.1002/bmc.258
Elkasabgy NA (2014) Ocular supersaturated self-nanoemulsifying drug delivery systems (S-SNEDDS) to enhance econazole nitrate bioavailability. Int J Pharm 460:33–44. https://doi.org/10.1016/j.ijpharm.2013.10.044
Trepanier DJ, Ure DR, Foster RT (2018) Development, characterization, and pharmacokinetic evaluation of a crv431 loaded self-microemulsifying drug delivery system. J Pharm Pharm Sci 21:335s–348s. https://doi.org/10.18433/jpps30245
Lim C, Kim Won D, Sim T et al (2016) Preparation and characterization of a lutein loading nanoemulsion system for ophthalmic eye drops. J Drug Deliv Sci Technol 36:168–174. https://doi.org/10.1016/j.jddst.2016.10.009
Rimple NMJ (2018) Impact of ocular compatible lipoids and castor oil in fabrication of brimonidine tartrate nanoemulsions by 3 3 full factorial design. Recent Pat Inflamm Allergy Drug Discov 12:169–183. https://doi.org/10.2174/1872213x12666180730115225
Mahboobian MM, Seyfoddin A, Aboofazeli R et al (2019) Brinzolamide–loaded nanoemulsions: ex vivo transcorneal permeation, cell viability and ocular irritation tests. Pharm Dev Technol 24:600–606. https://doi.org/10.1080/10837450.2018.1547748
Patel N, Nakrani H, Raval M, Sheth N (2016) Development of loteprednol etabonate-loaded cationic nanoemulsified in-situ ophthalmic gel for sustained delivery and enhanced ocular bioavailability. Drug Deliv 23:3712–3723. https://doi.org/10.1080/10717544.2016.1223225
Anton N, Vandamme TF (2011) Nano-emulsions and micro-emulsions: Clarifications of the critical differences. Pharm Res 28:978–985. https://doi.org/10.1007/s11095-010-0309-1
Bharti SK, Kesavan K (2017) Phase-transition W/O microemulsions for ocular delivery: evaluation of antibacterial activity in the treatment of bacterial keratitis. Ocul Immunol Inflamm 25:463–474. https://doi.org/10.3109/09273948.2016.1139136
Ameeduzzafar A, Ali J, Fazil M et al (2016) Colloidal drug delivery system: amplify the ocular delivery. Drug Deliv 23:710–726. https://doi.org/10.3109/10717544.2014.923065
De Souza JF, Maia KN, De Oliveira Patrício PS et al (2016) Ocular inserts based on chitosan and brimonidine tartrate: development, characterization and biocompatibility. J Drug Deliv Sci Technol 32:21–30. https://doi.org/10.1016/j.jddst.2016.01.008
Shah BM, Misra M, Shishoo CJ, Padh H (2015) Nose to brain microemulsion-based drug delivery system of rivastigmine: formulation and ex-vivo characterization. Drug Deliv 22:918–930
Rönkkö S, Vellonen KS, Järvinen K et al (2016) Human corneal cell culture models for drug toxicity studies. Drug Deliv Transl Res 6:660–675. https://doi.org/10.1007/s13346-016-0330-y
Silva MM, Calado R, Marto J et al (2017) Chitosan nanoparticles as a mucoadhesive drug delivery system for ocular administration. Mar Drugs 15:1–16. https://doi.org/10.3390/md15120370
Bhasker S, Kislay R, Rupinder KK, Jagat KR (2015) Evaluation of nanoformulated therapeutics in an ex-vivo bovine corneal irritation model. Toxicol Vitr 29:917–925. https://doi.org/10.1016/j.tiv.2015.01.007
Funding
The corresponding author would like to thank, Department of Science and Technology and SERB (INSPIRE Grant No: IFA-LSBM-13 and EMR/2016/007966/HS) for project funds and Ministry of Chemicals and Fertilizer for providing fellowship.
Author information
Authors and Affiliations
Contributions
MC: Conception, design of the work, acquisition and analysis of data, Initial draft preparation, data compilation. KN: Cell line experiments execution, Initial draft preparation, interpretation of results. NN and YN: Designing experimental studies, interpretation of histological data, technical inputs in drafting of manuscript. DK: Writing- Reviewing and Editing. MM: Concept design, interpretation, revision, and final approval for publication, accuracy integrity, accountability for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choudhari, M., Nayak, K., Nagai, N. et al. Role of mucoadhesive agent in ocular delivery of ganciclovir microemulsion: cytotoxicity evaluation in vitro and ex vivo. Int Ophthalmol 43, 1153–1167 (2023). https://doi.org/10.1007/s10792-022-02514-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-022-02514-z