Abstract
Purpose
This study aimed to evaluate the rate and risk factors for primary failure and recurrence after intravitreal anti-VEGF injection in retinopathy of prematurity (ROP).
Methods
This retrospective study was performed on 865 eyes from 441 patients with retinopathy of prematurity receiving intravitreal bevacizumab from 2012 to 2019. Medical records of patients were evaluated.
Results
Mean gestational age (GA) and birth weight of patients were 28 ± 2 weeks and 1121 ± 312 g, respectively. Thirty-five eyes (4.04%) had a primary failure, including 18 eyes from 187 eyes in zone 1 (9.6%) and 17 eyes from 678 eyes in zone 2 (2.5%). The mean time of retreatment was 16.64 ± 13.68 days in eyes without regression ROP. The remaining 830 eyes (95.95%) were included in recurrence analysis. The recurrence occurred in 33 eyes (3.97%) of them in 20 patients, with the meantime of 77.52 days after the first treatment (IVB). The presence of plus disease, history of oxygen therapy or phototherapy, and GA less than 32 were associated with significantly increased prevalence of treatment failure. The risk factors predicting recurrence are lower birth weight, zone 1 pretreatment, history of intubation, anemia, and sepsis.
Conclusion
Intravitreal anti-VEGF is a successful treatment for ROP with a low rate of primary failure and recurrence. Awareness of risk factors for treatment failure and recurrence may help clinicians to schedule more vigilant approach in susceptible cases.
Similar content being viewed by others
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Gillbert C, Muhit M (2008) Twenty years of childhood blindness: What have we learnt? Community Eye Heal J. 21(67):46–47. Accessed December 16, 2020. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2580065/
Hartnett ME, Penn JS (2012) Mechanisms and management of retinopathy of prematurity. N Engl J Med 367(26):2515–2526. https://doi.org/10.1056/NEJMra1208129
Good WV, Group ET for R of PC (2004) Final results of the early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 102:233–250. https://pubmed.ncbi.nlm.nih.gov/15747762
Geloneck MM, Chuang AZ, Clark WL et al (2014) Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol 132(11):1327–1333. https://doi.org/10.1001/jamaophthalmol.2014.2772
Mintz-Hittner HA, Kennedy KA, Chuang AZ (2011) Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 364(7):603–615. https://doi.org/10.1056/NEJMoa1007374
Roohipoor R, Karkhaneh R, Riazi-Esfahani M et al (2018) Comparison of intravitreal bevacizumab and laser photocoagulation in the treatment of retinopathy of prematurity. Ophthalmol Retin 2(9):942–948. https://doi.org/10.1016/j.oret.2018.01.017
Pertl L, Steinwender G, Mayer C et al (2015) A systematic review and meta-analysis on the safety of vascular endothelial growth factor (VEGF) inhibitors for the treatment of retinopathy of prematurity. PLoS One 10(6):e0129383. https://doi.org/10.1371/journal.pone.0129383
Bazvand F, Pour EK, Gharehbaghi G et al (2020) Hypertension and ischemic stroke after aflibercept for retinopathy of prematurity. Int Med Case Rep J 13:243–247. https://doi.org/10.2147/IMCRJ.S258881
Bazvand F, Riazi-Esfahani H, Mirshahi A et al (2021) Ocular complications following intravitreal bevacizumab injection for retinopathy of prematurity and assessment of risk factors. Int J Retin Vitr 7(1):5. https://doi.org/10.1186/s40942-020-00276-3
Hwang CK, Hubbard GB, Hutchinson AK, Lambert SR (2015) Outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity: a 5-year retrospective analysis. Ophthalmology 122(5):1008–1015. https://doi.org/10.1016/j.ophtha.2014.12.017
Hoang QV, Kiernan DF, Chau FY, Shapiro MJ, Blair MP (2010) Fluorescein angiography of recurrent retinopathy of prematurity after initial intravitreous bevacizumab treatment. Arch Ophthalmol 128(8):1080–1081. https://doi.org/10.1001/archophthalmol.2010.145
Hajrasouliha AR, Garcia-Gonzales JM, Shapiro MJ, Yoon H, Blair MP (2017) Reactivation of retinopathy of prematurity three years after treatment with bevacizumab. Ophthalmic Surg Lasers Imaging Retina 48(3):255–259. https://doi.org/10.3928/23258160-20170301-10
Mintz-Hittner HA, Geloneck MM, Chuang AZ (2016) Clinical management of recurrent retinopathy of prematurity after intravitreal bevacizumab monotherapy. Ophthalmology 123(9):1845–1855. https://doi.org/10.1016/j.ophtha.2016.04.028
Ling KP, Liao PJ, Wang NK et al (2020) Rates and risk factors for recurrence of retinopathy of prematurity after laser or intravitreal anti-vascular endothelial growth factor monotherapy. Retina 40(9):1793–1803. https://doi.org/10.1097/IAE.0000000000002663
Martínez-Castellanos MA, González-H León A, Romo-Aguas JC, Gonzalez-Gonzalez LA (2020) A proposal of an algorithm for the diagnosis and treatment of recurrence or treatment failure of retinopathy of prematurity after anti-VEGF therapy based on a large case series. Graefe’s Arch Clin Exp Ophthalmol = Albr von Graefes Arch fur Klin und Exp Ophthalmol 258(4):767–772. https://doi.org/10.1007/s00417-020-04605-y
Chen TA, Shields RA, Bodnar ZH, Callaway NF, Schachar IH, Moshfeghi DM (2019) A spectrum of regression following intravitreal bevacizumab in retinopathy of prematurity. Am J Ophthalmol 198:63–69. https://doi.org/10.1016/j.ajo.2018.09.039
Karkhaneh R, Khodabande A, Riazi-Eafahani M et al (2016) Efficacy of intravitreal bevacizumab for zone-II retinopathy of prematurity. Acta Ophthalmol 94(6):e417–e420. https://doi.org/10.1111/aos.13008
Snyder LL, Garcia-Gonzalez JM, Shapiro MJ, Blair MP (2016) Very late reactivation of retinopathy of prematurity after monotherapy with intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging Retina 47(3):280–283. https://doi.org/10.3928/23258160-20160229-12
Hu J, Blair MP, Shapiro MJ, Lichtenstein SJ, Galasso JM, Kapur R (2012) Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol (Chicago, Ill 1960) 130(8):1000–1006. https://doi.org/10.1001/archophthalmol.2012.592
Campbell JP, Ataer-Cansizoglu E, Bolon-Canedo V et al (2016) Expert diagnosis of plus disease in retinopathy of prematurity from computer-based image analysis. JAMA Ophthalmol 134(6):651–657. https://doi.org/10.1001/jamaophthalmol.2016.0611
Chan JJT, Lam CPS, Kwok MKM et al (2016) Risk of recurrence of retinopathy of prematurity after initial intravitreal ranibizumab therapy. Sci Rep 6(1):1–7. https://doi.org/10.1038/srep27082
Schaffer DB, Palmer EA, Plotsky DF et al (1993) Prognostic factors in the natural course of retinopathy of prematurity. Ophthalmology 100(2):230–237. https://doi.org/10.1016/S0161-6420(93)31665-9
Alajbegovic-Halimic J, Zvizdic D, Alimanovic-Halilovic E, Dodik I, Duvnjak S (2015) Risk factors for retinopathy of prematurity in premature born children. Med Arch (Sarajevo, Bosnia Herzegovina) 69(6):409–413. https://doi.org/10.5455/medarh.2015.69.409-413
Allegaert K, Casteels I, Cossey V, Devlieger H (2003) Retinopathy of prematurity: any difference in risk factors between a high and low risk population? Eur J Ophthalmol 13(9–10):784–788. https://doi.org/10.1177/1120672103013009-1009
Chen YH, Lien RI, Tsai S et al (2015) Natural history of retinopathy of prematurity: two-year outcomes of a prospective study. Retina 35(1):141–148. https://doi.org/10.1097/IAE.0000000000000270
Giapros V, Drougia A, Asproudis I, Theocharis P, Andronikou S (2011) Low gestational age and chronic lung disease are synergistic risk factors for retinopathy of prematurity. Early Hum Dev 87(10):653–657. https://doi.org/10.1016/j.earlhumdev.2011.05.003
Lyu J, Zhang Q, Chen C-LL et al (2017) Recurrence of retinopathy of prematurity after intravitreal ranibizumab monotherapy: timing and risk factors. Invest Ophthalmol Vis Sci 58(3):1719–1725. https://doi.org/10.1167/iovs.16-20680
Hu Q, Bai Y, Chen X, Huang L, Chen Y, Li X (2017) Recurrence of retinopathy of prematurity in zone II stage 3+ after ranibizumab treatment: a retrospective study. J Ophthalmol 2017:1–5. https://doi.org/10.1155/2017/5078565
Choi RY, Brown JM, Kalpathy-Cramer J et al (2020) Variability in plus disease identified using a deep learning-based retinopathy of prematurity severity scale. Ophthalmol Retina 4(10):1016–1021. https://doi.org/10.1016/j.oret.2020.04.022
Taylor S, Brown JM, Gupta K et al (2019) Monitoring disease progression with a quantitative severity scale for retinopathy of prematurity Using deep learning. JAMA Ophthalmol 137(9):1022–1028. https://doi.org/10.1001/jamaophthalmol.2019.2433
Campbell JP, Kim SJ, Brown JM et al (2020) Evaluation of a novel retinopathy of prematurity severity scale applied by clinicians and deep learning. Ophthalmology. https://doi.org/10.1016/j.ophtha.2020.10.025
Kinsey VE (1956) Retrolental fibroplasia; cooperative study of retrolental fibroplasia and the use of oxygen. AMA Arch Ophthalmol 56(4):481–543
Flynn JT, Bancalari E, Snyder ES et al (1992) A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. N Engl J Med 326(16):1050–1054. https://doi.org/10.1056/NEJM199204163261603
Cunningham S, Mclntosh N, Fleck BW, Elton RA (1995) Transcutaneous oxygen levels in retinopathy of prematurity. Lancet 346(8988):1464–1465. https://doi.org/10.1016/S0140-6736(95)92475-2
Bossi E, Koerner F, Flury B, Zulauf M (1984) Retinopathy of prematurity: a risk factor analysis with univariate and multivariate statistics. Helv Paediatr Acta 39(4):307–317
BOOST II United Kingdom Collaborative Group, BOOST II Australia Collaborative Group, BOOST II New Zealand Collaborative Group, Stenson BJ, Tarnow-Mordi WO, Darlow BA, et al. (2013) Oxygen Saturation and Outcomes in Preterm Infants. N Engl J Med 368(22):2094–2104. https://doi.org/10.1056/nejmoa1302298
Darlow BA, Hutchinson JL, Henderson-Smart DJ, Donoghue DA, Simpson JM, Evans NJ (2005) Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand neonatal network. Pediatrics 115(4):990–996. https://doi.org/10.1542/peds.2004-1309
Zarei M, Bazvand F, Ebrahimiadib N et al (2019) Prevalence and risk factors of retinopathy of prematurity in Iran. J Ophthalmic Vis Res 14(3):291–298. https://doi.org/10.18502/jovr.v14i3.4785
Mitchell AJ, Green A, Jeffs DA, Roberson PK (2011) Physiologic effects of retinopathy of prematurity screening examinations. Adv Neonatal Care 11(4):291–297. https://doi.org/10.1097/ANC.0b013e318225a332
Acknowledgements
Special thanks to Mehdi Yaseri PhD for data analysis.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
FB and HRE involved in conception and design, definition of intellectual content, data acquisition, analysis and interpretation, manuscript review, and guarantor. KF and MH and MMB involved in analysis and interpretation, literature search, data acquisition, manuscript preparation, manuscript editing, and manuscript review. AA and ADF involved in definition of intellectual content, literature search and manuscript review, consistent criticism. All authors read and approved the final manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethical approval
Human subjects were included in this study. The ethics committee of eye research center, Farabi Eye Hospital, approved this study (https://ethics.research.ac.ir/IR.TUMS.FARABI.REC.1399.040).
Consent to participate
The study was conducted according to the tenets of the Declaration of Helsinki. Any procedure was done after providing parents with informed consent.
Consent for publication
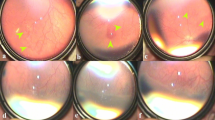
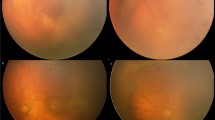
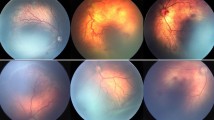
Written informed consent was obtained from the patient’s parents (that his fundus photographs were used in the Figue1) for publication of any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fadakar, K., Mehrabi Bahar, M., Riazi-Esfahani, H. et al. Intravitreal bevacizumab to treat retinopathy of prematurity in 865 eyes: a study to determine predictors of primary treatment failure and recurrence. Int Ophthalmol 42, 2017–2028 (2022). https://doi.org/10.1007/s10792-021-02198-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-02198-x