Abstract
Key messages
The pathogenesis of subretinal neovascularization (SRNV) due to macular telengiectasia (MacTel 2) has not fully elucidated. This optical coherence tomography (OCT)-based method can provide better understanding of the pathogenesis of SRNV due to MacTel 2.
Purpose
To evaluate the choroidal vascular index (CVI) through optical coherence tomography (OCT) on eyes with proliferative macular telangiectasia type 2 (MacTel 2) or non-proliferative MacTel 2, and in healthy individuals.
Methods
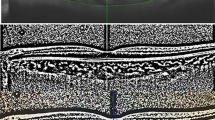
Macular enhanced depth imaging OCT scans on 42 eyes of 21 patients with non-proliferative MacTel 2, on 32 eyes of 20 patients with proliferative MacTel 2, and on 38 eyes of 32 control patients were analyzed by adjusting for age–gender–axial length. Proliferative MacTel 2 was diagnosed when subretinal neovascularization (SRNV) was simultaneously observed in the non-proliferative phase. Binarization methods of ImageJ software were used to analyze images, and total choroid area (TCA), luminal area (LA) and stromal area (SA) were obtained. CVI was characterized as the ratio of LA to TCA.
Results
The mean TCA and SA were significantly higher in group 1 and group 2 when compared with group 3 (3.36 ± 0.29 mm2 vs. 3.27 ± 0.76 mm2 vs. 2.49 ± 0.24 mm2, p < 0.001; 1.15 ± 0.31 mm2 vs. 1.10 ± 0.69 mm2 vs. 0.35 ± 0.23 mm2, respectively; p < 0.001). Although LA was relatively higher in group 1 and group 2 than group 3, no statistically significant difference was observed (2.22 ± 0.14 mm2 vs. 2.17 ± 0.15 mm2 vs. 2.13 ± 0.21 mm2) (p = 0.088). CVI was significantly lower in group 1 than other groups (0.65 ± 0.01 vs 0.67 ± 0.02 vs 0.68 ± 0.02) (p < 0.001).
Conclusion
As an OCT screening method, CVI may be used to assess the vascular status of the choroid on the eyes which are naive for or were exposed to SRNV secondary to MacTel 2, and to elucidate the pathogenesis of this disease.




Similar content being viewed by others
References
Gass JDOR (1982) Idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol 100:769–780
Gass JDBB (1993) Idiopathic juxtafoveolar retinal telangiectasis. Update of classification and follow-up study. Ophthalmology 100:1536–1546
Charbel Issa PHF, Scholl HP (2007) Findings in fluorescein angiography and optical coherence tomography after intravitreal bevacizumab in type 2 idiopathic macular telangiectasia. Ophthalmology 114(9):1736–1742
Narayanan RMA, Hussain N, Hussain A, Jalali S (2008) Characterization of idiopathic macular telangiectasia type 2 by fundus fluorescein angiography in Indian population. Eur J Ophthalmol 18(4):587–590
Koizumi HIT, Maruko I (2006) Morphologic features of group 2A idiopathic juxtafoveolar retinal telangiectasis in three-dimensional optical coherence tomography. Am J Ophthalmol 142(2):340–343
Wu LET, Arevalo JF (2013) Idiopathic macular telangiectasia type 2 (idiopathic juxtafoveolar retinal telangiectasis type 2A, Mac Tel 2). Surv Ophthalmol 58:536–559
Potter MJSS, Chan EY, Morris AHC (2002) Photodynamic therapy of a subretinal neovascular membrane in type 2A idiopathic juxtafoveolar retinal telangiectasis. Am J Ophthalmol 133(1):149–151
Clemons TE, Gillies MC, Chew EY, Bird AC et al (2010) Baseline characteristics of participants in the natural history study of macular telangiectasia (MacTel) MacTel project report no. 2. Ophthalmic Epidemiol 17(1):66–73
Chhablani JKI, Jonnadula GB, Venkata A, Narayanan R (2014) Choroidal thickness in macular telangiectasia type 2. Retina 34(9):1819–1823
Nunes RP, GoldhardtdeAmorimGarciaFilho RCA, Thorell MR et al (2015) Spectral-domain optical coherence tomography measurements of choroidal thickness and outer retinal disruption in macular telangiectasia type 2. Ophthalmic Surg Las Imaging Retina 46(2):162–170
Iovino C, Pellegrini M, Bernabei F et al (2020) Choroidal vascularity index: an in-depth analysis of this novel optical coherence tomography parameter. J Clin Med 9(2):595
Endo H, Kase S, Saito M et al (2020) Choroidal thickness in diabetic patients without diabetic retinopathy: a meta-analysis. Am J Ophthalmol 218:68–77
Alışık M, Işik MU (2020) The relationship between choroidal thickness and intracellular oxidised-reduced glutathione and extracellular thiol-disulfide homeostasis at different stages of diabetic retinopathy. Curr Eye Res 10:1–6
Agrawal RGP, Tan KA, Cheung CMG, Wong TY (2016) Choroidal vascularity index as a measure of vascular status of the choroid: measurements in healthy eyes from a populationbased study. Sci Rep 6:21090
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Bland JM, Altman DG (2012) Agreed statistics: measurement method comparison. Anesthesiology 116:182–185
Kumar VKD, Kumar P (2019) Swept source optical coherence tomography analysis of choroidal thickness in macular telangiectasia type 2: a case-control study. Graefes Arch Clin Exp Ophthalmol 257(3):567–573
Heeren TFCKD, Florea D, Clemons TE et al (2018) Longitudinal correlation of ellipsoid zone loss and functional loss in macular telangiectasia type 2. Retina 38(Suppl 1):S20–S26
Kupitz EHHT, Holz FG, Charbel Issa P (2015) Poor long-term outcome of anti-vascular endothelial growth factor therapy in nonproliferative macular telangiectasia type 2. Retina 35(12):2619–2626
Wang JC, Laíns I, Oellers P, Kim IK (2019) Choroidal thickness and vascular density in macular telangiectasia type 2 using en face swept-source optical coherence tomography. Br J Ophthalmol 103(11):1584–1589
Len ACPM, Zhu L, Hageman GS, Song X (2012) Pilot application of iTRAQ to the retinal disease macular telangiectasia. J Proteome Res 11(2):537–553
LA Yannuzzi BA, Freund KB, Chen KJ, Eandi CM (2006) Idiopathic macular telangiectasia. Arch Ophthalmol 124(4):450–460
Sallo FBPT, Egan C, Wolf-Schnurrbusch UE et al (2012) En face OCT imaging of the IS/OS junction line in type 2 idiopathic macular telangiectasia. Invest Ophthalmol Vis Sci 53(10):6145–6152
Linsenmeier RAP-SL (2000) Metabolic dependence of photoreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci 41:3117–3123
LA Yannuzzi BA, Freund KB, Chen KJ, Eandi CM (2012) Idiopathic macular telangiectasia 2006. Retina 32(Suppl 1):450–460
Newman ERA (1996) The Müller cell: a functional element of the retina. Trends Neurosci 19:307–312
Okada M, Egan CA, Heeren TFC, Tufail A (2018) Macular telangiectasia type 2: quantitative analysis of a novel phenotype and implications for the pathobiology of the disease. Retina 38(Suppl 1):S97–S104
Tout SC-LT, Hollander H, Stone J (1993) The role of muller cells in the formation of the blood-retinal barrier. Neuroscience 55:291–301
Gass JDM (1997) Stereoscopic atlas of macular diseases: diagnosis and treatment., vol 1. 4 Mosby, St Louis
Shen W, Fruttiger M, Zhu L et al (2012) Conditional müller cell ablation causes independent neuronal and vascular pathologies in a novel transgenic model. J Neurosci 32(45):15715–15727
Zhang QWR, Chen CL, Legarreta AD et al (2015) Swept source optical coherence tomography angiography of neovascular macular telangiectasia type 2. Retina 35(11):2285–2299
Melrose MAML, Goldberg RE, Annesley WH Jr (1987) Subretinal neovascular membranes associated with choroidal nonperfusion and retinal ischemia. Ann Ophthalmol 19:396–399
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Bugra Karasu declares that he has no conflict of interest. Author Ali Rıza Cenk Celebi declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained prior to every surgical procedure from all individual participants included in the study. We would like to thank Editage (www.editage.com) for English language editing.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karasu, B., Celebi, A.R.C. Choroidal vascularity index: an enhanced depth optical coherence tomography-based parameter to determine vascular status in patients with proliferative and non-proliferative macular telangiectasia. Int Ophthalmol 41, 3505–3513 (2021). https://doi.org/10.1007/s10792-021-01917-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-01917-8