Abstract
Purpose
The aim of this study was to employ newly developed advanced image analysis software to evaluate changes in retinal layer thickness following hemodialysis.
Methods
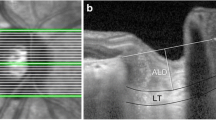
A non-randomized prospective study of patients with end-stage renal disease assessed on the same day before and after hemodialysis. Intraocular pressure and central corneal thickness were analyzed, and spectral domain optical coherence tomography results were automatically segmented using the Orion software and then compared. All patients had normal retinal optical coherence tomography findings before hemodialysis.
Results
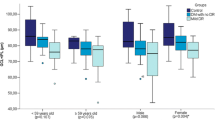
Of the 31 suitable end-stage renal disease patients treated with hemodialysis who provided consent to participate, seven were unable to complete all evaluations, leaving 24 patients for analysis in the final study group. Their mean age was 66.67±14.3 years (range: 35-88), and 62.5% were males. Mean central corneal thickness did not change following hemodialysis (563.4±30.2 µm to 553.1±47.2 µm, p=.247), while mean intraocular pressure decreased (14.48±2.5 mmHg to 13.16±2.28 mmHg, p=.028). Individual mean retinal layer thickness showed no significant change, including the retinal nerve fiber layer (40.9±6.8 µm to 40.1±5.2 µm, p=.412), the ganglion cell and the inner plexiform layer (68.66±8 µm to 69.03±7.6 µm, p=.639), and the photoreceptor layer (50.26±2.8 µm to 50.32±3.1 µm, p=.869). Total retinal thickness similarly remained constant, with a mean of 303.7±17.3 µm before and 304.33±18.4 µm after hemodialysis (p=.571).
Conclusions
Thickness of retinal layers, as assessed by individual segmentation, and central corneal thickness were not affected by hemodialysis treatment, while intraocular pressure was significantly reduced among patients with end-stage renal disease without pre-existing ocular pathology who were undergoing hemodialysis. These results support the view that hemodialysis does not have a negative impact on the retinal morphology of end-stage renal disease patients, who comprise a population with high rates of diabetic and/or hypertensive retinopathy as well as vision-threatening complications.
Similar content being viewed by others
Data availability
All data and material are available upon reasonable request from the corresponding author.
References
Cheung N, Mitchell P, Wong TY (2010) Diabetic retinopathy. Lancet 376(9735):124–136. https://doi.org/10.1016/S0140-6736(09)62124-3
Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjornsdottir S, Wedel H, Clements M, Dahlqvist S, Lind M (2015) Excess mortality among persons with type 2 diabetes. New Engl J Med 373(18):1720–1732. https://doi.org/10.1056/NEJMoa1504347
Theodossiadis PG, Theodoropoulou S, Neamonitou G, Grigoropoulos V, Liarakos V, Triantou E, Theodossiadis GP, Vlahakos DV (2012) Hemodialysis-induced alterations in macular thickness measured by optical coherence tomography in diabetic patients with end-stage renal disease. Ophthalmol J Int d’ophtalmol Int J Ophthalmol 227(2):90–94. https://doi.org/10.1159/000331321
Pahor D, Gracner B, Gracner T, Hojs R (2008) Optical coherence tomography findings in hemodialysis patients. Klin Monbl Augenheilkd 225(8):713–717. https://doi.org/10.1055/s-2007-963761
Perkovich BT, Meyers SM (1988) Systemic factors affecting diabetic macular edema. Am J Ophthalmol 105(2):211–212
Bresnick GH (1983) Diabetic maculopathy. A critical review highlighting diffuse macular edema. Ophthalmology 90 (11):1301–1317
Jung JW, Yoon MH, Lee SW, Chin HS (2013) Effect of hemodialysis (HD) on intraocular pressure, ocular surface, and macular change in patients with chronic renal failure Effect of hemodialysis on the ophthalmologic findings. Graefes Arch Clin Exp Ophthalmol 251(1):153–162. https://doi.org/10.1007/s00417-012-2032-6
Emre S, Ozturkeri A, Ulusoy MO, Cankurtaran C (2016) Evaluation of the acute effect of haemodialysis on retina and optic nerve with optical coherence tomography. Saudi J Ophthalmol 30(4):233–235. https://doi.org/10.1016/j.sjopt.2016.10.007
Azem N, Spierer O, Shaked M, Neudorfer M (2014) Effect of hemodialysis on retinal thickness in patients with diabetic retinopathy, with and without macular edema. Using Optical Coherence Tomography J Ophthalmol 2014:709862. https://doi.org/10.1155/2014/709862
Kal A, Kal O, Eroglu FC, Oner O, Kucukerdonmez C, Yilmaz G (2016) Evaluation of choroidal and retinal thickness measurements in adult hemodialysis patients using spectral-domain optical coherence tomography. Arq Bras Oftalmol 79(4):229–232. https://doi.org/10.5935/0004-2749.20160066
Chen H, Zhang X, Shen X (2018) Ocular changes during hemodialysis in patients with end-stage renal disease. BMC Ophthalmol 18(1):208. https://doi.org/10.1186/s12886-018-0885-0
Gronenschild EH, Habets P, Jacobs HI, Mengelers R, Rozendaal N, van Os J, Marcelis M (2012) The effects of freesurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS ONE 7(6):e38234. https://doi.org/10.1371/journal.pone.0038234
Keller J, Oakley JD, Russakoff DB, Andorra-Ingles M, Villoslada P, Sanchez-Dalmau BF (2016) Changes in macular layers in the early course of non-arteritic ischaemic optic neuropathy. Graefe’s Arch Clin Exp Ophthalmol 254(3):561–567. https://doi.org/10.1007/s00417-015-3066-3
Hecht I, Yeshurun I, Bartov E, Bar A, Burgansky-Eliash Z, Achiron A (2018) Retinal layers thickness changes following epiretinal membrane surgery. Eye (Lond) 32(3):555–562
Daugirdas JT, Depner TA, Inrig J, Mehrotra R, Rocco MV, Suri RS, Weiner DE, Greer N, Ishani A, MacDonald R, Olson C, Rutks I, Slinin Y, Wilt TJ, Rocco M, Kramer H, Choi MJ, Samaniego-Picota M, Scheel PJ, Willis K, Joseph J, Brereton L (2015) KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis 66(5):884–930. https://doi.org/10.1053/j.ajkd.2015.07.015
Karakosta A, Vassilaki M, Plainis S, Elfadl NH, Tsilimbaris M, Moschandreas J (2012) Choice of analytic approach for eye-specific outcomes: one eye or two? Am J Ophthalmol 153(3):571-579.e571
Tan J, Yang Y, Jiang H, Liu C, Deng Z, Lam BL, Hu L, Oakley J, Wang J (2016) The measurement repeatability using different partition methods of intraretinal tomographic thickness maps in healthy human subjects. Clin Ophthalmol 10:2403–2415. https://doi.org/10.2147/OPTH.S117494
Seigo MA, Sotirchos ES, Newsome S, Babiarz A, Eckstein C, Ford E, Oakley JD, Syc SB, Frohman TC, Ratchford JN, Balcer LJ, Frohman EM, Calabresi PA, Saidha S (2012) In vivo assessment of retinal neuronal layers in multiple sclerosis with manual and automated optical coherence tomography segmentation techniques. J Neurol 259(10):2119–2130. https://doi.org/10.1007/s00415-012-6466-x
Hammes HP, Welp R, Kempe HP, Wagner C, Siegel E, Holl RW, Mellitus DPVI-GBCND (2015) Risk factors for retinopathy and dme in type 2 diabetes-results from the german/austrian dpv database. PLoS ONE 10(7):e0132492. https://doi.org/10.1371/journal.pone.0132492
Boelter MC, Gross JL, Canani LH, Costa LA, Lisboa HR, Tres GS, Lavinsky J, Azevedo MJ (2006) Proliferative diabetic retinopathy is associated with microalbuminuria in patients with type 2 diabetes. Braz J Med Biol Res 39(8):1033–1039
Doshi SM, Friedman AN (2017) Diagnosis and management of type 2 diabetic kidney disease. Clin J Am Soc Nephrol 12(8):1366–1373. https://doi.org/10.2215/CJN.11111016
Liang S, Le W, Liang D, Chen H, Xu F, Chen H, Liu Z, Zeng C (2016) Clinico-pathological characteristics and outcomes of patients with biopsy-proven hypertensive nephrosclerosis: a retrospective cohort study. BMC Nephrol 17:42. https://doi.org/10.1186/s12882-016-0254-2
Tokuyama T, Ikeda T, Sato K (1998) Effect of plasma colloid osmotic pressure on intraocular pressure during haemodialysis. Br J Ophthalmol 82(7):751–753
Sitprija V, Holmes JH, Ellis PP (1964) Changes in intraocular pressure during hemodialysis. Invest Ophthalmol 3:273–284
Acknowledgements
Esther Eshkol, MA, medical and scientific copyeditor, is thanked for editorial assistance
Funding
The authors received no financial support for the research, authorship, and/or publication of this article. Use of the Orion® software was provided free of charge by Voxeleron LLC.
Author information
Authors and Affiliations
Contributions
IM and LF conceived and directed the project. AA, OS, ZB, AB, AG, AK, and RC recruited patients, analyzed data, and critically revised the manuscript. IH, IM, AA, and LM analyzed data and drafted the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were in accordance with the Wolfson medical center research committee and in accordance with the 1964 Helsinki declaration and its later amendments.
Consent to publish
All participants agreed to the collection and anonymous publication of the data.
Informed consent to participate
Informed written consent was obtained from all participants prior to enrollment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maharshak, I., Hecht, I., Mankuta, L. et al. The effect of hemodialysis on individual retinal layer thickness. Int Ophthalmol 41, 1233–1240 (2021). https://doi.org/10.1007/s10792-020-01677-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-020-01677-x