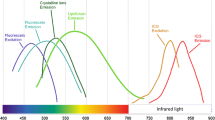
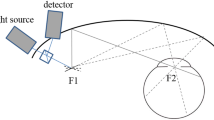
Abstract
Purpose
To review the basic principles of ultra-widefield fundus autofluorescence (UWF-FAF) and discuss its clinical application for a variety of retinal and choroidal disorders.
Methods
A systematic review of the PubMed database was performed using the search terms “ultra-widefield,” “autofluorescence,” “retinal disease” and “choroidal disease.”
Results
UWF-FAF imaging is a recently developed noninvasive retinal imaging modality with a wide imaging range that can locate peripheral fundus lesions that traditional fundus autofluorescence cannot. Multiple commercially available ultra-widefield imaging systems, including Heidelberg Spectralis and Optomap Ultra-Widefield systems, are available to the clinician. Imaging by UWF-FAF is more comprehensive; it can reflect the content and distribution of the predominant ocular fluorophore in retinal pigment epithelial cells and evaluate the metabolic status of RPE of various retinal and choroidal disorders.
Conclusion
UWF-FAF can detect abnormalities that traditional fundus autofluorescence cannot; therefore, it can be used to better elucidate disease pathogenesis, analyze genotype–phenotype correlations, diagnose and monitor disease.







Similar content being viewed by others
References
Delori FC, Dorey CK, Staurenghi G, Arend O, Goger DG, Weiter JJ (1995) In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci 36:718–729
von Ruckmann A, Fitzke FW, Bird AC (1995) Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br J Ophthalmol 79:407–412
Delori FC, Goger DG, Dorey CK (2001) Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci 42:1855–1866
Spital G, Radermacher M, Muller C, Brumm G, Lommatzsch A, Pauleikhoff D (1998) Autofluorescence characteristics of lipofuscin components in different forms of late senile macular degeneration. Klin Monbl Augenheilkd 213:23–31
Weiter JJ, Delori FC, Wing GL, Fitch KA (1986) Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest Ophthalmol Vis Sci 27:145–152
Schutt F, Davies S, Kopitz J, Holz FG, Boulton ME (2000) Photodamage to human RPE cells by A2-E, a retinoid component of lipofuscin. Invest Ophthalmol Vis Sci 41:2303–2308
Bergmann M, Schutt F, Holz FG, Kopitz J (2004) Inhibition of the ATP-driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2-E may contribute to the pathogenesis of age-related macular degeneration. FASEB J 18:562–564
Maeda A, Golczak M, Chen Y, Okano K, Kohno H, Shiose S, Ishikawa K, Harte W, Palczewska G, Maeda T, Palczewski K (2011) Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat Chem Biol 8:170–178
Roberts JE, Kukielczak BM, Hu DN, Miller DS, Bilski P, Sik RH, Motten AG, Chignell CF (2002) The role of A2E in prevention or enhancement of light damage in human retinal pigment epithelial cells. Photochem Photobiol 75:184–190
Crouch RK, Koutalos Y, Kono M, Schey K, Ablonczy Z (2015) A2E and lipofuscin. Prog Mol Biol Transl Sci 134:449–463
Ablonczy Z, Higbee D, Anderson DM, Dahrouj M, Grey AC, Gutierrez D, Koutalos Y, Schey KL, Hanneken A, Crouch RK (2013) Lack of correlation between the spatial distribution of A2E and lipofuscin fluorescence in the human retinal pigment epithelium. Invest Ophthalmol Vis Sci 54:5535–5542
Smith RT, Bernstein PS, Curcio CA (2013) Rethinking A2E. Invest Ophthalmol Vis Sci 54:5543
Feeney L (1978) Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Invest Ophthalmol Vis Sci 17:583–600
Feeney-Burns L, Hilderbrand ES, Eldridge S (1984) Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci 25:195–200
Keilhauer CN, Delori FC (2006) Near-infrared autofluorescence imaging of the fundus: visualization of ocular melanin. Invest Ophthalmol Vis Sci 47:3556–3564
Kayatz P, Thumann G, Luther TT, Jordan JF, Bartz-Schmidt KU, Esser PJ, Schraermeyer U (2001) Oxidation causes melanin fluorescence. Invest Ophthalmol Vis Sci 42:241–246
Hu DN, Simon JD, Sarna T (2008) Role of ocular melanin in ophthalmic physiology and pathology. Photochem Photobiol 84:639–644
Burke JM, Kaczara P, Skumatz CM, Zareba M, Raciti MW, Sarna T (2011) Dynamic analyses reveal cytoprotection by RPE melanosomes against non-photic stress. Mol Vis 17:2864–2877
Wang Z, Dillon J, Gaillard ER (2006) Antioxidant properties of melanin in retinal pigment epithelial cells. Photochem Photobiol 82:474–479
Rozanowski B, Burke JM, Boulton ME, Sarna T, Rozanowska M (2008) Human RPE melanosomes protect from photosensitized and iron-mediated oxidation but become pro-oxidant in the presence of iron upon photodegradation. Invest Ophthalmol Vis Sci 49:2838–2847
Sundelin SP, Nilsson SE, Brunk UT (2001) Lipofuscin-formation in cultured retinal pigment epithelial cells is related to their melanin content. Free Radic Biol Med 30:74–81
Sarna T (1992) Properties and function of the ocular melanin–a photobiophysical view. J Photochem Photobiol B 12:215–258
Nicolas CM, Robman LD, Tikellis G, Dimitrov PN, Dowrick A, Guymer RH, McCarty CA (2003) Iris colour, ethnic origin and progression of age-related macular degeneration. Clin Exp Ophthalmol 31:465–469
Weiter JJ, Delori FC, Wing GL, Fitch KA (1985) Relationship of senile macular degeneration to ocular pigmentation. Am J Ophthalmol 99:185–187
Tso MO (1981) Pathology and pathogenesis of drusen of the optic nervehead. Ophthalmology 88:1066–1080
Friedman AH, Beckerman B, Gold DH, Walsh JB, Gartner S (1977) Drusen of the optic disc. Surv Ophthalmol 21:373–390
Heussen FM, Tan CS, Sadda SR (2012) Prevalence of peripheral abnormalities on ultra-widefield greenlight (532 nm) autofluorescence imaging at a tertiary care center. Invest Ophthalmol Vis Sci 53:6526–6531
Ben Moussa N, Georges A, Capuano V, Merle B, Souied EH, Querques G (2015) MultiColor imaging in the evaluation of geographic atrophy due to age-related macular degeneration. Br J Ophthalmol 99:842–847
Witmer MT, Parlitsis G, Patel S, Kiss S (2013) Comparison of ultra-widefield fluorescein angiography with the Heidelberg Spectralis((R)) noncontact ultra-widefield module versus the Optos((R)) Optomap((R)). Clin Ophthalmol 7:389–394
Staurenghi G, Viola F, Mainster MA, Graham RD, Harrington PG (2005) Scanning laser ophthalmoscopy and angiography with a wide-field contact lens system. Arch Ophthalmol 123:244–252
Cicinelli MV, Cavalleri M, Brambati M, Lattanzio R, Bandello F (2019) New imaging systems in diabetic retinopathy. Acta Diabetol. https://doi.org/10.1007/s00592-019-01373-y
Shin JY, Choi HJ, Lee J, Choi M, Chung B, Byeon SH (2016) Fundus autofluorescence findings in central serous chorioretinopathy using two different confocal scanning laser ophthalmoscopes: correlation with functional and structural status. Graefes Arch Clin Exp Ophthalmol 254:1537–1544
Bonnay G, Nguyen F, Meunier I, Ducasse A, Hamel C, Arndt C (2011) Screening for retinal detachment using wide-field retinal imaging. J Fr Ophtalmol 34:482–485
Klein R, Chou CF, Klein BE, Zhang X, Meuer SM, Saaddine JB (2011) Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol 129:75–80
Holz FG, Bellman C, Staudt S, Schutt F, Volcker HE (2001) Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci 42:1051–1056
Sepah YJ, Akhtar A, Sadiq MA, Hafeez Y, Nasir H, Perez B, Mawji N, Dean DJ, Ferraz D, Nguyen QD (2014) Fundus autofluorescence imaging: fundamentals and clinical relevance. Saudi J Ophthalmol 28:111–116
Nomura Y, Takahashi H, Tan X, Obata R, Yanagi Y (2015) Widespread choroidal thickening and abnormal midperipheral fundus autofluorescence characterize exudative age-related macular degeneration with choroidal vascular hyperpermeability. Clin Ophthalmol 9:297–304
Witmer MT, Kozbial A, Daniel S, Kiss S (2012) Peripheral autofluorescence findings in age-related macular degeneration. Acta Ophthalmol 90:e428–e433
Tan CS, Heussen F, Sadda SR (2013) Peripheral autofluorescence and clinical findings in neovascular and non-neovascular age-related macular degeneration. Ophthalmology 120:1271–1277
Writing Committee for the OPRs, Domalpally A, Clemons TE, Danis RP, Sadda SR, Cukras CA, Toth CA, Friberg TR, Chew EY (2017) Peripheral retinal changes associated with age-related macular degeneration in the age-related eye disease study 2: age-related eye disease study 2 report number 12 by the age-related eye disease study 2 optos peripheral retina (OPERA) study research group. Ophthalmology 124:479–487
Forshaw TRJ, Minor AS, Subhi Y, Sorensen TL (2019) Peripheral retinal lesions in eyes with age-related macular degeneration using ultra-widefield imaging: a systematic review with meta-analyses. Ophthalmol Retina. https://doi.org/10.1016/j.oret.2019.04.014
Duisdieker V, Fleckenstein M, Zilkens KM, Steinberg JS, Holz FG, Schmitz-Valckenberg S (2015) Long-term follow-up of fundus autofluorescence imaging using wide-field scanning laser ophthalmoscopy. Ophthalmologica 234:218–226
Klufas MA, Yannuzzi NA, Pang CE, Srinivas S, Sadda SR, Freund KB, Kiss S (2015) Feasibility and clinical utility of ultra-widefield indocyanine green angiography. Retina 35:508–520
Nicholson B, Noble J, Forooghian F, Meyerle C (2013) Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol 58:103–126
Iacono P, Battaglia PM, Papayannis A, La Spina C, Varano M, Bandello F (2015) Acute central serous chorioretinopathy: a correlation study between fundus autofluorescence and spectral-domain OCT. Graefes Arch Clin Exp Ophthalmol 253:1889–1897
Imamura Y, Fujiwara T, Spaide RF (2011) Fundus autofluorescence and visual acuity in central serous chorioretinopathy. Ophthalmology 118:700–705
Pang CE, Shah VP, Sarraf D, Freund KB (2014) Ultra-widefield imaging with autofluorescence and indocyanine green angiography in central serous chorioretinopathy. Am J Ophthalmol 158(362–371):e2
Reznicek L, Seidensticker F, Stumpf C, Kampik A, Thurau S, Kernt M, Neubauer A (2014) Systematic analysis of wide-field fundus autofluorescence (FAF) imaging in posterior uveitis. Curr Eye Res 39:164–171
Heussen FM, Vasconcelos-Santos DV, Pappuru RR, Walsh AC, Rao NA, Sadda SR (2011) Ultra-wide-field green-light (532-nm) autofluorescence imaging in chronic Vogt-Koyanagi-Harada disease. Ophthalmic Surg Lasers Imaging 42:272–277
Mesquida M, Llorenc V, Fontenla JR, Navarro MJ, Adan A (2014) Use of ultra-wide-field retinal imaging in the management of active Behcet retinal vasculitis. Retina 34:2121–2127
Hashimoto H, Kishi S (2015) Ultra-wide-field fundus autofluorescence in multiple evanescent white dot syndrome. Am J Ophthalmol 159:698–706
Seidensticker F, Neubauer AS, Wasfy T, Stumpf C, Thurau SR, Kampik A, Kernt M (2011) Wide-field fundus autofluorescence corresponds to visual fields in chorioretinitis patients. Clin Ophthalmol 5:1667–1671
Oishi A, Oishi M, Ogino K, Morooka S, Yoshimura N (2016) Wide-field fundus autofluorescence for retinitis pigmentosa and cone/cone-rod dystrophy. Adv Exp Med Biol 854:307–313
Lorenz B, Wabbels B, Wegscheider E, Hamel CP, Drexler W, Preising MN (2004) Lack of fundus autofluorescence to 488 nanometers from childhood on in patients with early-onset severe retinal dystrophy associated with mutations in RPE65. Ophthalmology 111:1585–1594
Ogura S, Yasukawa T, Kato A, Usui H, Hirano Y, Yoshida M, Ogura Y (2014) Wide-field fundus autofluorescence imaging to evaluate retinal function in patients with retinitis pigmentosa. Am J Ophthalmol 158:1093–1098
Hariri AH, Gui W, Datoo O’Keefe GA, Ip MS, Sadda SR, Gorin MB (2018) Ultra-widefield fundus autofluorescence imaging of patients with retinitis pigmentosa: a standardized grading system in different genotypes. Ophthalmol Retina 2:735–745
Oishi M, Oishi A, Ogino K, Makiyama Y, Gotoh N, Kurimoto M, Yoshimura N (2014) Wide-field fundus autofluorescence abnormalities and visual function in patients with cone and cone-rod dystrophies. Invest Ophthalmol Vis Sci 55:3572–3577
Oishi A, Ogino K, Makiyama Y, Nakagawa S, Kurimoto M, Yoshimura N (2013) Wide-field fundus autofluorescence imaging of retinitis pigmentosa. Ophthalmology 120:1827–1834
Trichonas G, Traboulsi EI, Ehlers JP (2016) Correlation of ultra-widefield fundus autofluorescence patterns with the underlying genotype in retinal dystrophies and retinitis pigmentosa. Ophthalmic Genet. https://doi.org/10.1080/13816810.2016.1227450:1-5
Allikmets R (1997) A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet 17:122
Kjellstrom U (2014) Association between genotype and phenotype in families with mutations in the ABCA4 gene. Mol Vis 20:89–104
Allikmets R, Shroyer NF, Singh N et al (1997) Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science 277:1805–1807
Klufas MA, Tsui I, Sadda SR, Hosseini H, Schwartz SD (2017) Ultrawidefield autofluoresence in Abca4 stargardt disease. Retina. https://doi.org/10.1097/IAE.0000000000001567
Kumar V (2017) Insights into autofluorescence patterns in Stargardt macular dystrophy using ultra-wide-field imaging. Graefes Arch Clin Exp Ophthalmol. https://doi.org/10.1007/s00417-017-3736-4
Abalem MF, Otte B, Andrews C, Joltikov KA, Branham K, Fahim AT, Schlegel D, Qian CX, Heckenlively JR, Jayasundera T (2017) Peripheral visual fields in ABCA4 stargardt disease and correlation with disease extent on ultra-widefield fundus autofluorescence. Am J Ophthalmol 184:181–188
Shen LL, Sun M, Grossetta Nardini HK, Del Priore LV (2019) Natural history of autosomal recessive stargardt disease in untreated eyes: a systematic review and meta-analysis of study- and individual-level data. Ophthalmology. https://doi.org/10.1016/j.ophtha.2019.05.015
Nagiel A, Lalane RA, Sadda SR, Schwartz SD (2016) Ultra-widefield fundus imaging: a review of clinical applications and future trends. Retina 36:660–678
Bell DJ, Wilson MW (2004) Choroidal melanoma: natural history and management options. Cancer Control 11:296–303
Albert DM, Robinson NL, Fulton AB et al (1980) Epidemikological investigation of increased incidence of choroidal melanoma in a single population of chemical workers. Int Ophthalmol Clin 20:71–92
Kernt M, Schaller UC, Stumpf C, Ulbig MW, Kampik A, Neubauer AS (2010) Choroidal pigmented lesions imaged by ultra-wide-field scanning laser ophthalmoscopy with two laser wavelengths (Optomap). Clin Ophthalmol 4:829–836
Reznicek L, Stumpf C, Seidensticker F, Kampik A, Neubauer AS, Kernt M (2014) Role of wide-field autofluorescence imaging and scanning laser ophthalmoscopy in differentiation of choroidal pigmented lesions. Int J Ophthalmol 7:697–703
Shields JA (1978) Melanocytoma of the optic nerve head: a review. Int Ophthalmol 1:31–37
Krohn J, Kjersem B (2011) Stereo fundus photography in the diagnosis of optic disc melanocytoma. Acta Ophthalmol 89:e533–e534
Arroyo JG, Yang L, Bula D, Chen DF (2005) Photoreceptor apoptosis in human retinal detachment. Am J Ophthalmol 139:605–610
Meyerle CB, Smith RT, Barbazetto IA, Yannuzzi LA (2007) Autofluorescence of basal laminar drusen. Retina 27:1101–1106
Albertus DL, Schachar IH, Zahid S, Elner VM, Demirci H, Jayasundera T (2013) Autofluorescence quantification of benign and malignant choroidal nevomelanocytic tumors. JAMA Ophthalmol 131:1004–1008
Salvanos P, Utheim TP, Moe MC, Eide N, Bragadomicronttir R (2015) Autofluorescence imaging in the differential diagnosis of optic disc melanocytoma. Acta Ophthalmol 93:476–480
Kadonosono K, Itoh N, Uchio E, Nakamura S, Ohno S (2000) Staining of internal limiting membrane in macular hole surgery. Arch Ophthalmol 118:1116–1118
Eckardt C, Eckert T, Eckardt U, Porkert U, Gesser C (2008) Macular hole surgery with air tamponade and optical coherence tomography-based duration of face-down positioning. Retina 28:1087–1096
Ciardella AP, Lee GC, Langton K, Sparrow J, Chang S (2004) Autofluorescence as a novel approach to diagnosing macular holes. Am J Ophthalmol 137:956–959
Shiragami C, Shiraga F, Nitta E, Fukuda K, Yamaji H (2012) Correlation of increased fundus autofluorescence signals at closed macula with visual prognosis after successful macular hole surgery. Retina 32:281–288
Nakao S, Arita R, Sato Y et al (2016) Wide-field laser ophthalmoscopy for imaging of gas-filled eyes after macular hole surgery. Clin Ophthalmol 10:1623–1630
Cook B, Lewis GP, Fisher SK, Adler R (1995) Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci 36:990–996
Hagimura N, Iida T, Suto K, Kishi S (2002) Persistent foveal retinal detachment after successful rhegmatogenous retinal detachment surgery. Am J Ophthalmol 133:516–520
Heimann H, Zou X, Jandeck C et al (2006) Primary vitrectomy for rhegmatogenous retinal detachment: an analysis of 512 cases. Graefes Arch Clin Exp Ophthalmol 244:69–78
Witmer MT, Cho M, Favarone G, Chan RV, D’Amico DJ, Kiss S (2012) Ultra-wide-field autofluorescence imaging in non-traumatic rhegmatogenous retinal detachment. Eye (Lond) 26:1209–1216
Salvanos P, Navaratnam J, Ma J, Bragadottir R, Moe MC (2013) Ultra-widefield autofluorescence imaging in the evaluation of scleral buckling surgery for retinal detachment. Retina 33:1421–1427
Singer M, Sagong M, van Hemert J, Kuehlewein L, Bell D, Sadda SR (2016) Ultra-widefield imaging of the peripheral retinal vasculature in normal subjects. Ophthalmology 123:1053–1059
Acknowledgements
We would like to thank Dr. Schwartz SD, Dr. SriniVas R. Sadda, and Dr. Panagiotis Salvanos for their contributions to the photographs shown in this paper, and Dr. Michael A. Klufas for providing the data shown in the table.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no relevant financial or scientific conflicts of interests to disclose in this manuscript.
Ethical approval
All the procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. And this paper does not contain any studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, A., Chen, C. Clinical application of ultra-widefield fundus autofluorescence. Int Ophthalmol 41, 727–741 (2021). https://doi.org/10.1007/s10792-020-01609-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-020-01609-9