Abstract
Purpose
The purpose of this study was to evaluate the accuracy of the convolutional neural network (CNN) model in glaucoma identification with three primary colors (red, green, blue; RGB) and split color channels using fundus photographs with a small sample size.
Methods
The dataset was prepared using color fundus photographs captured with a fundus camera (VX-10i, Kowa Co., Ltd., Tokyo, Japan). The training dataset consisted of 200 images, and the validation dataset contained 60 images. In the preprocessing stage, the color channels for the fundus images were separated into red (red model), green (green model), and blue (blue model) using OpenCV on Windows. All images were resized to squares with a size of 512 × 512 pixels for preprocessing before input into the model, and the model was fine-tuned with VGG16.
Results
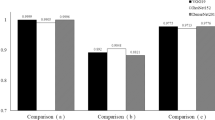
The diagnostic performance was significantly higher in the green model [area under the curve (AUC) 0.946; 95% confidence interval (CI) 0.851–0.982] than in the RGB model (AUC 0.800; 95% CI 0.658–0.893; P = 0.006), red model (AUC 0.746; 95% CI 0.601–0.851; P = 0.002), and blue model (AUC 0.558; 95% CI 0.405–0.700; P < 0.001).
Conclusion
The present study showed that the green digital filter is useful for structuring CNN models for automatic discrimination of glaucoma using fundus photographs with a small sample size. The present findings suggest that preprocessing, when creating the CNN model, is an important step for the identification of a large number of retinal diseases using color fundus photographs.







Similar content being viewed by others
References
Quigley HA (2011) Glaucoma. Lancet 377(9774):1367–1377. https://doi.org/10.1016/S0140-6736(10)61423-7
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121(11):2081–2090. https://doi.org/10.1016/j.ophtha.2014.05.013
Chan WC, Morin JD, McCulloch C (1976) Optic disc observations in glaucoma. Can J Ophthalmol 11(2):134–139
Townsend KA, Wollstein G, Schuman JS (2009) Imaging of the retinal nerve fibre layer for glaucoma. Br J Ophthalmol 93(2):139–143. https://doi.org/10.1136/bjo.2008.145540
Myers JS, Fudemberg SJ, Lee D (2018) Evolution of optic nerve photography for glaucoma screening: a review. Clin Exp Ophthalmol 46(2):169–176. https://doi.org/10.1111/ceo.13138
Bussel II, Wollstein G, Schuman JS (2014) OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol 98:15–19. https://doi.org/10.1136/bjophthalmol-2013-304326
Alexandrescu C, Dascalu AM, Panca A, Sescioreanu A, Mitulescu C, Ciuluvica R, Voinea L, Celea C (2010) Confocal scanning laser ophthalmoscopy in glaucoma diagnosis and management. J Med Life 3(3):229–234
Vilupuru AS, Rangaswamy NV, Frishman LJ, Smith EL, Harwerth RS, Roorda A (2007) Adaptive optics scanning laser ophthalmoscopy for in vivo imaging of lamina cribrosa. J Opt Soc Am A 24(5):1417–1425. https://doi.org/10.1364/Josaa.24.001417
Takayama K, Ooto S, Hangai M, Ueda-Arakawa N, Yoshida S, Akagi T, Ikeda HO, Nonaka A, Hanebuchi M, Inoue T, Yoshimura N (2013) High-resolution imaging of retinal nerve fiber bundles in glaucoma using adaptive optics scanning laser ophthalmoscopy. Am J Ophthalmol 155(5):870–881. https://doi.org/10.1016/j.ajo.2012.11.016
Shibata N, Tanito M, Mitsuhashi K, Fujino Y, Matsuura M, Murata H, Asaoka R (2018) Development of a deep residual learning algorithm to screen for glaucoma from fundus photography. Sci Rep 8(1):14665. https://doi.org/10.1038/s41598-018-33013-w
Krizhevsky A, Sutskever I, Hinton GE (2017) ImageNet classification with deep convolutional neural networks. Commun ACM 60(6):84–90. https://doi.org/10.1145/3065386
Ting DSW, Cheung CYL, Lim G, Tan GSW, Quang ND, Gan A, Hamzah H, Garcia-Franco R, Yeo IYS, Lee SY, Wong EYM, Sabanayagam C, Baskaran M, Ibrahim F, Tan NC, Finkelstein EA, Lamoureux EL, Wong IY, Bressler NM, Sivaprasad S, Varma R, Jonas JB, He MG, Cheng CY, Cheung GCM, Aung T, Hsu W, Lee ML, Wong TY (2017) Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA, J Am Med Assoc 318(22):2211–2223. https://doi.org/10.1001/jama.2017.18152
Li Z, He Y, Keel S, Meng W, Chang RT, He M (2018) Efficacy of a deep learning system for detecting glaucomatous optic neuropathy based on color fundus photographs. Ophthalmology 125(8):1199–1206. https://doi.org/10.1016/j.ophtha.2018.01.023
Son J, Shin JY, Kim HD, Jung KH, Park KH, Park SJ (2020) Development and validation of deep learning models for screening multiple abnormal findings in retinal fundus images. Ophthalmology 127(1):85–94. https://doi.org/10.1016/j.ophtha.2019.05.029
Phene S, Dunn RC, Hammel N, Liu Y, Krause J, Kitade N, Schaekermann M, Sayres R, Wu DJ, Bora A, Semturs C, Misra A, Huang AE, Spitze A, Medeiros FA, Maa AY, Gandhi M, Corrado GS, Peng L, Webster DR (2019) Deep learning and glaucoma specialists the relative importance of optic disc features to predict glaucoma referral in fundus photographs. Ophthalmology 126(12):1627–1639. https://doi.org/10.1016/j.ophtha.2019.07.024
Inoue H (2018) Data augmentation by pairing samples for images classification. arXiv:1801.02929
Wong SC, Gatt A, Stamatescu V, McDonnell MD (2016) Understanding data augmentation for classification: when to warp? arXiv:1609.08764v08762
Bennett TJ, Barry CJ (2009) Ophthalmic imaging today: an ophthalmic photographer’s viewpoint—a review. Clin Exp Ophthalmol 37(1):2–13. https://doi.org/10.1111/j.1442-9071.2008.01812.x
Ahn JM, Kim S, Ahn KS, Cho SH, Lee KB, Kim US (2018) A deep learning model for the detection of both advanced and early glaucoma using fundus photography. PLoS ONE 13(11):e0207982. https://doi.org/10.1371/journal.pone.0207982
Japan Glaucoma Society. Guidlines for Glaucoma. http://www.ryokunaisho.jp/english/guidelines.html. Accessed 16 June 2019
He K, Zhang X, Ren S, Sun J (2015) Deep residual learning for image recognition. arXiv:1512.03385
Szegedy C, Vanhoucke V, Loffe S, Shlens J, Wojna Z (2015) Rethinking the inception architecture for computer vision. arXiv:1512.00567
Li M, Yang Y, Jiang H, Gregori G, Roisman L, Zheng F, Ke BL, Qu DY, Wang JH (2017) Retinal microvascular network and microcirculation assessments in high myopia. Am J Ophthalmol 174:56–67. https://doi.org/10.1016/j.ajo.2016.10.018
Selvaraju R, Cogswell M, Das A, Vedantam R, Parikh D, Batra D (2017) Grad-CAM: visual explanations from deep networks via gradient-based localization. arXiv:1610.02391v02393
Geeraets WJ, Williams RC, Chan G, Ham WT Jr, Guerry D 3rd, Schmidt FH (1962) The relative absorption of thermal energy in retina and choroid. Invest Ophthalmol 1:340–347
Lee J, Kim Y, Kim JH, Park KH (2019) Screening glaucoma with red-free fundus photography using deep learning classifier and polar transformation. J Glaucoma 28(3):258–264. https://doi.org/10.1097/Ijg.0000000000001187
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, Grp EMGT (2002) Reduction of intraocular pressure and glaucoma progression—results from the early manifest glaucoma trial. Arch Ophthalmol Chic 120(10):1268–1279. https://doi.org/10.1001/archopht.120.10.1268
Prata TS, De Moraes CG, Teng CC, Tello C, Ritch R, Liebmann JM (2010) Factors affecting rates of visual field progression in glaucoma patients with optic disc hemorrhage. Ophthalmology 117(1):24–29. https://doi.org/10.1016/j.ophtha.2009.06.028
Ohno-Matsui K, Kawasaki R, Jonas JB, Cheung CM, Saw SM, Verhoeven VJ, Klaver CC, Moriyama M, Shinohara K, Kawasaki Y, Yamazaki M, Meuer S, Ishibashi T, Yasuda M, Yamashita H, Sugano A, Wang JJ, Mitchell P, Wong TY, Group ME-afPMS (2015) International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol 159(5):877–883. https://doi.org/10.1016/j.ajo.2015.01.022
Acknowledgements
This study was supported by grants from Japan Society for the Promotion of Science, Grants-in-Aid for Scientific Research (KAKENHI multi-year Fund), Grant Number of 19K20728 and Santen Pharmaceutical (Grant No. 6).
Author information
Authors and Affiliations
Contributions
Substantial contributions are as follows: MH, AM contributed for the conception and design; KI for the acquisition of data; MH for analysis and interpretation of data and drafting the article or revising it critically for important intellectual content. MH, AM, TM, TH, JK, TS, KI contributed for the final approval of the version to be published, and MH, AM, TM, TH, JK, TS, KI for the agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This investigation adheres to the tenets of the World Medical Association Declaration of Helsinki. The experimental protocols and consent procedures were approved by the Institutional Review Board of Teikyo University (approval no. 18-161).
Informed consent
Informed consent was obtained from all subjects after explaining the nature and possible complications of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hirota, M., Mizota, A., Mimura, T. et al. Effect of color information on the diagnostic performance of glaucoma in deep learning using few fundus images. Int Ophthalmol 40, 3013–3022 (2020). https://doi.org/10.1007/s10792-020-01485-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-020-01485-3