Abstract
Purpose
To compare in vivo swept-source optical coherence tomography (SS-OCT) measurements of the ciliary muscle (CM) in patients with primary open-angle glaucoma (POAG) and healthy subjects, and examine correlations between CM dimensions and several covariates.
Methods
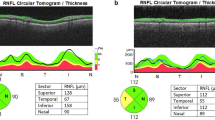
This was a cross-sectional study of the right eyes of 181 subjects: 89 POAG patients and 92 healthy subjects. Using the Triton SS-OCT device (Topcon, Tokyo, Japan), CM length (CML), area (CMA) and thickness measured 1000 µm (CMT1), 2000 µm (CMT2) and 3000 µm (CMT3) from the scleral spur were determined in the temporal and nasal quadrants. POAG patients were subjected to visual field (VF) and peripapillary retinal nerve fiber layer (RNFL) assessment. CM dimensions were then assessed for correlation with VF mean defect (MD), mean RNFL thickness and intraocular pressure (IOP).
Results
Mean CMLs were 4325 ± 340 µm and 4195 ± 843 µm for the healthy subjects and POAG patients, respectively (p = 0.17). Mean CM thicknesses were CMT1 = 546 ± 56 µm, CMT2 = 326 ± 44 µm and CMT3 = 174 ± 16 µm in the healthy eyes versus CMT1 = 534 ± 108, CMT2 = 332 ± 99 and CMT3 = 183 ± 74 in the POAG eyes, with no significant differences detected (all p ≥ 0.25). In the temporal quadrant, mean CMA was 1.12 ± 0.29 mm2 and 1.15 ± 0.24 mm2 for the healthy and POAG subjects, respectively (p = 0.45). No correlations were observed between CM measurements and RNFL thickness (p ≥ 0.15), IOP or VF MD (p ≥ 0.14) in POAG subjects irrespective of glaucoma severity (p ≥ 0.19).
Conclusions
While SS-OCT proved useful to measure CM dimensions in vivo, these dimensions did not differ between healthy individuals and POAG subjects. In the patients with POAG, no correlations were detected between CM dimensions and VF, RNFL or IOP.


Similar content being viewed by others
References
Sheppard AL, Davies LN (2010) In vivo analysis of ciliary muscle morphologic changes with accommodation and axial ametropia. Invest Ophthalmol Vis Sci 51:6882–6889
Lewis HA, Kai C, Sinnott LT, Bailey MD (2012) Changes in ciliary muscle thickness during accommodation in children. Optom Vis Sci 89:727–737
Lossing LA, Sinnott LT, Kai C, Richdale K, Bailey MD (2012) Measuring changes in ciliary muscle thickness with accommodation in young adults. Optom Vis Sci 89:719–726
Sheppard AL, Davies LN (2011) The effect of ageing on in vivo human ciliary muscle morphology and contractility. Invest Ophthalmol Vis Sci 52:1809–1816
Mao Y, Bai HX, Li B, Xu XL, Gao F, Zhang ZB, Jonas JB (2018) Dimensions of the ciliary muscles of Brücke, Müller and Iwanoff and their associations with axial length and glaucoma. Graefes Arch Clin Exp Ophthalmol 256:2165–2171
Croft MA, Lütjen-Drecoll E, Kaufman PL (2017) Age-related posterior ciliary muscle restriction—a link between trabecular meshwork and optic nerve head pathophysiology. Exp Eye Res 158:187–189
Ku JY, Nongpiur ME, Park J, Narayanaswamy AK, Perera SA, Tun TA, Kumar RS, Baskaran M, Aung T (2014) Qualitative evaluation of the iris and ciliary body by ultrasound biomicroscopy in subjects with angle closure. J Glaucoma 23:583–588
Lindsey JD, Kashiwagi K, Kashiwagi F et al (1997) Prostaglandins alter extracellular matrix adjacent to human ciliary muscle cells in vitro. Invest Ophthalmol Vis Sci 38:2214–2223
Marchini G, Babighian S, Tosi R et al (1999) Effects of 0.2% brimonidine on ocular anterior structures. J Ocul Pharmacol Ther 15:337–344
Bailey MD, Sinnott LT, Mutti DO (2008) Ciliary body thickness and refractive error in children. Invest Ophthalmol Vis Sci 49:4352–4360
Jeon S, Lee WK, Lee K, Moon NJ (2012) Diminished ciliary muscle movement on accommodation in myopia. Exp Eye Res 105:9–14
Pucker AD, Sinnott LT, Kao C, Bailey MD (2013) Region specific relationships between refractive error and ciliary muscle thickness in children. Invest Ophthalmol Vis Sci 54:4710–4716
Lütjen-Drecoll E, Shimizu T, Rohrbach M, Rohen JW (1986) Quantitative analysis of ‘plaque material’ between ciliary muscle tips in normal and glaucomatous eyes. Exp Eye Res 42:457–465
Gao K, Li F, Li Y, Li X, Huang W, Chen S, Liu Y, Aung T, Zhang X (2018) Anterior choroidal thickness increased in primary open-angle glaucoma and primary angle-closure disease eyes evidenced by ultrasound biomicroscopy and SS-OCT. Invest Ophthalmol Vis Sci 59:1270–1277
Aiello AL, Tran VT, Rao NA (1992) Postnatal development of the ciliary body and pars plana. Arch Ophthalmol 110:802–805
Esteve-Taboada JJ, Domínguez-Vicent A, Monsálvez-Romín D, Del Águila-Carrasco AJ, Montés-Micó R (2017) Non-invasive measurements of the dynamic changes in the ciliary muscle, crystalline lens morphology, and anterior chamber during accommodation with a high-resolution OCT. Graefes Arch Clin Exp Ophthalmol 255:1385–1394
Ruggeri M, de Freitas C, Williams S, Hernandez VM, Cabot F, Yesilirmak N, Alawa K, Chang YC, Yoo SH, Gregori G, Parel JM, Manns F (2016) Quantification of the ciliary muscle and crystalline lens interaction during accommodation with synchronous OCT imaging. Biomed Opt Express 7:1351–1364
Pardue MT, Sivak JG (2000) Age-related changes in human ciliary muscle. Optom Vis Sci 77:204–210
Domínguez-Vicent A, Monsálvez-Romín D, Esteve-Taboada JJ, Montés-Micó R, Ferrer-Blasco T (2019) Effect of age in the ciliary muscle during accommodation: sectorial analysis. J Optom 12:14–21
Ang M, Baskaran M, Werkmeister RM, Chua J, Schmidl D, Aranha Dos Santos V, Garhöfer G, Mehta JS, Schmetterer L (2018) Anterior segment optical coherence tomography. Prog Retin Eye Res 66:132–156
Laughton DS, Coldrick BJ, Sheppard AL, Davies LN (2015) A program to analyse optical coherence tomography images of the ciliary muscle. Cont. Lens Anterior Eye 38:402–408
Fernandez-Vigo JI, Shi H, Kudsieh B, Arriola-Villalobos P, De-Pablo Gómez-de-Liaño L, García-Feijóo J, Fernández-Vigo JA (2019) Ciliary muscle dimensions by swept-source optical coherence tomography and correlation study in a large population. Acta Ophthalmol. https://doi.org/10.1111/aos.14304
Bailey MD (2011) How should we measure the ciliary muscle? Invest Ophthalmol Vis Sci 52:1817–1818
Wang Z, Chung C, Lin J, Xu J, Huang J (2016) Quantitative measurements of the ciliary body in eyes with acute primary-angle closure. Invest Ophthalmol Vis Sci 57:3299–3305
Marchini G, Ghilotti G, Bonadimani M, Babighian S (2003) Effects of 0.005% latanoprost on ocular anterior structures and ciliary body thickness. J Glaucoma 12:295–300
Oliveira C, Tello C, Liebmann JM, Ritch R (2005) Ciliary body thickness increases with increasing axial myopia. Am J Ophthalmol 140:324–325
Li X, Wang W, Huang W, Chen S, Wang J, Wang Z, Liu Y, He M, Zhang X (2018) Difference of uveal parameters between the acute primary angle closure eyes and the fellow eyes. Eye (Lond) 32:1174–1182
Výborný P, Hejsek L, Sicáková S, Pasta J (2007) Changes of the thickness of the ciliary body after the latanoprost 0.005% application. Cesk Slov Oftalmol 63:418–421
Funding
No financial support was received for this submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the other authors has a conflict of interest with this submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kudsieh, B., Fernández-Vigo, J.I., Shi, H. et al. Ciliary muscle dimensions measured by swept-source optical coherence tomography in eyes with primary open-angle glaucoma and healthy eyes. Int Ophthalmol 40, 2247–2255 (2020). https://doi.org/10.1007/s10792-020-01405-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-020-01405-5