Abstract
Introduction
Thyroid eye disease (TED) is the most frequent extra-thyroid manifestation of Graves’ disease and it is more frequent in middle age and in female gender. Nowadays, the causal mechanisms of this disease are not completely understood, but the current available studies suggest that the main causative factor is the thyroid stimulating hormone receptor.
Materials and methods
To collect reports on TED medical management, a thorough literature search was performed in PubMed database. An additional search was made in Google Scholar to complete the collected items.
Results
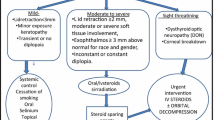
Among the indentified risk factors, tobacco habit is the most relevant. The main criteria to choose a suitable treatment are the activity and severity of the disease. Support measures can be used to improve the patient’s symptoms in any phase of the disease. There is a large number of drugs proposed to manage TED, although with different reported rates of success.
Conclusions
Currently, the drugs of choice are corticosteroids in moderate-to-severe and in sight-threatening forms. The main problem of corticosteroids is their spectrum of side effects. Therefore, other alternatives are being suggested for medical management of this disease. The efficacy of these alternatives remains unclear.
Similar content being viewed by others
References
De Groot L, Chrousos G, Dungan K et al (2000) Graves’ disease and the manifestations of thyrotoxicosis. Endotext, South Dartmouth
Abraham-Nordling M, Byström K, Törring O, Lantz M, Berg G, Calissendorf J et al (2011) Incidence of hyperthyroidism in Sweden. Eur J Endocrinol 165:899–905
Barrio-Barrio J, Sabater A, Bonet-Farriol E, Velázquez-Villoria Á, Galofré J (2015) Graves’ ophthalmopathy: VISA vs EUGOGO classification, assessment, and management. J Ophthalmol 2015:249125
Bartalena L, Baldeschi L, Bobodoris K, Eckstein A, Kahaly G, Marcocci C et al (2016) The 2016 European thyroid association/European Group on Graves’ Orbitopathy guidelines for the management of Graves’ orbitopathy. Eur Thyroid 5:9–26
Briceño C, Gupta S, Douglas R (2013) Advances in the management of thyroid eye disease. Int Ophthalmol 53:93–101
Rivera-Grana E, Lin P, Suhler E, Rosenbaum J (2015) Methotrexate as a corticosteroid-sparing agent for thyroid eye disease. J Clin Exp Ophthalmol 6:422
Bartalena L (2013) Graves’ orbitopathy: imperfect treatments for a rare disease. Eur Thyroid J. 2:259–269
Laurberg P, Berman D, Bülow Pedersen I, Andersen S, Carlé A (2012) Incidence and clinical presentation of moderate to severe graves’ orbitopathy in a Danish population before and after iodine fortification of salt. J Clin Endocrinol Metab 97:2325–2332
McKeag D, Lane C, Lazarus J, Baldeschi L, Boboridis K, Dickinson A et al (2007) Clinical features of dysthyroid optic neuropathy: a European Group on Graves’ Orbitopathy (EUGOGO) survey. Br J Ophthalmol 91:455–458
Wiersinga W (2007) Advances in treatment of active, moderate-to-severe Graves’ ophthalmopathy. Diabetes Endocrinol 5:134–142
Inaba H, Martin W, De Groot A, Qin S, De Groot L (2006) Thyrotropin receptor epitopes and their relation to histocompatibility leukocyte antigen-DR molecules in Graves’ disease. J Clin Endocrinol Metab 91:2286–2294
Akamizu T, Mori T, Nakao K (1997) Pathogenesis of Graves’ disease: molecular analysis of anti-thyrotropin receptor antibodies. Endocr J 44:633–646
Inaba H, De Groot L, Akamizu T (2016) Thyrotropin receptor epitope and human leukocyte antigen in Graves’ disease. Front Endocrinol (Lausanne) 7:120
Iyer S, Bahn R (2012) Immunopathogenesis of Graves’ ophthalmopathy: the role of the TSH receptor. Pract Res Clin Endocrinol Metab 26:281–289
Tsui S, Naik V, Hoa N, Hwang C, Afifiyan N, Sinha Hikim A et al (2008) Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol 181:4387–4405
Douglas R, Gianoukakis A, Kamat S, Smith T (2007) Aberrant expression of the insulin-like growth factor-1 receptor by T cells from patients with Graves’ disease may carry functional consequences for disease pathogenesis. J Immunol 178:3281–3287
Douglas R, Naik V, Hwang C, Afifiyan N, Gianoukakis A, Sand D et al (2008) B cells from patients with Graves’ disease aberrantly express the IGF-1 receptor: implications for disease pathogenesis. J Immunol 181:5768–5774
Smith T, Kahaly G, Ezra D, Fleming J, Dailey R, Tang R et al (2017) Teprotumumab for thyroid-associated ophthalmopy. N Engl J Med 376:1748–1761
Khong J, McNab A, Ebeling P, Craig J, Selva D (2016) Pathogenesis of thyroid eye disease: review and update on molecular mechanisms. Br J Ophthalmol 100:142–150
Mackay F, Browning J (2002) BAFF: a fundamental survival factor for B cells. Nat Rev Immunol 2:465–475
Lied G, Berstad A (2011) Functional and clinical aspects of the B-cell-activating factor (BAFF): a narrative review. Scand J Immunol 73:1–7
Leandro M, Cambridge G (2013) Expression of B cell activating factor (BAFF) and BAFF-biding receptors in rheumatoid arthritis. J Rheumathol 40:1247–1250
Theodorou E, Nezos A, Antypa E, Ioakeimidis D, Koutsilieris M, Tektonidou M et al (2018) B-cell activating factor and related genetic variants in lupus related atherosclerosis. J Autoimmun 92:87–92
Zhao Y, Li J, Wei B, Xu Z (2015) BAFF level increased in patients with autoimmune hemolytic anemia. Int J Clin Exp Med 8:2876–3882
van Steensel L, Dik W (2010) The orbital fibroblast: a key player and target for therapy in Graves’ ophthalmopathy. Orbit 29:202–206
van Steensel L, Hooijkaas H, Paridaens D, van de Bosch W, Kuijpers R, Drexhage H et al (2012) PDGF enhances orbital fibroblast responses to TSHR stimulating autoantibodies in Graves’ ophthalmopathy patients. J Clin Endocrinol Metab 97:944–953
Virakul S, van Steensel L, Dalm V, Paridaens D, van Hagen P, Dik W (2014) Platelet-derived growth factor: a key factor in the pathogenesis of Graves’ ophthalmopathy and potential target for treatment. Eur Thyroid 3:217–226
Dik W, Virakul S, van Steensel L (2016) Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves’ ophthalmopathy. Exp Eye Res 142:83–91
Bahn R (2010) Graves’ ophthalmopathy. N Engl J Med 362:726–738
Smith T (2010) Pathogenesis of Graves’ orbitopathy: a 2010 update. J Endocrinol 33:414–421
Mourist M, Prummel M, Wiersinga W, Koornneef L (1997) Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 47:9–14
Campi I, Vannucchi G, Salvi M (2016) Therapy of endocrine disease: endocrine dilemma: management of Graves’ orbitopathy. Eur J Endocrinol 175:117–133
Salvi M (2012) EUGOGO Atlas: EUGOGO protocol for assessment of Graves’ orbitopathy and completion of case record form Milan: EUGOGO
Werner S (1969) Classification of the eye changes of Graves’ disease. Am J Ophthalmol 68:646–648
Werner S (1977) Modification of the classification of the eye changes of Graves’ disease. Am J Ophthalmol 83:725–727
Prummel M, Wiersinga W (1993) Smoking and risk of Graves’ disease. JAMA 169:479–482
Wiersinga W (2013) Smoking and thyroid. Clin Endocrinol (Oxf) 79:145–151
Bartalena L, Marcocci C, Tanda M, Manetti L, Dell’Unto E, Bartolomei M et al (1998) Cigarette smoking and treatment outcomes in Graves ophthalmopathy. Ann Intern Med 129:632–635
Pfeilschifter J, Ziegler R (1996) Smoking and endocrine ophthalmopathy: impact of smoking severity and current vs lifetime cigarette consumption. Clin Endocrinol (Oxf) 45:477–481
Eckstein A, Quadbeck B, Mueller G, Rettenmeier A, Hoermann R, Mann K et al (2003) Impact of smoking on the response to treatment of thyroid associated ophthalmopathy. Br J Ophthalmol 87:773–776
Bartalena L (2012) Prevention of Graves’ ophthalmopathy. Best Pract Res Clin Endocrinol Metab 26:371–379
Krassas G, Segni M, Wiersinga W (2005) Childhood Graves’ ophthalmopathy: results of a European questionnaire study. Eur J Endocrinol 153:515–521
Eckstein A, Lösch C, Glowacka D, Schott M, Mann K, Esser J et al (2009) Euthyroid and primarily hypothyroid patients develop milder and significantly more asymmetrical Graves’ ophthalmopathy. Br J Ophthalmol 93:1052–1056
Termote K, Decallonne B, Mombaerts I (2014) The influence of prior hyperthyroidism on euthyroid Graves’ ophthalmopathy. J Ophthalmol 22:426898
Perros P, Hegedüs L, Bartalena L, Marcocci C, Kahaly G, Baldeschi L et al (2017) Graves’ orbitopathy are a rare disease in Europe: a European Group on Graves’ Orbitopathy (EUGOGO) position statement. Orphanet J Rare Dis 12:72
Bartley G, Fatourechi V, Kadrmas E, Jacobsen S, Ilstrup D, Garrity J et al (1996) Clinical features of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol 121:284–290
Bartalena L, Marccocci C, Bogazzi F, Panicucci M, Lepri A, Pinchera A (1989) Use of corticosteroids to prevent progression of Graves’ ophthalmopathy after radioiodine therapy for hyperthyroidism. N Engl J Med 321:1349–1352
Bartalena L, Marcocci C, Bogazzi F, Manetti L, Tanda M, Dell’Unto E et al (1998) Relation between therapy for hyperthyroidism and the course of Graves’ ophthalmopathy. N Engl J Med 338:73–78
Lai A, Sassi L, Compri E, Marino F, Sivelli P, Piantanida E et al (2010) Lower dose prednisone prevents radioiodine-associated exacerbation of initially mild or absent graves’ orbitopathy: a retrospective cohort study. J Clin Endocrinol Med 95:1333–1337
Negro R, Attanasio R, Grimaldi F, Guglielmi R, Papini E, AME (Associazione Medici Endocrinology) et al (2016) A 2015 Italian survey of clinical practice patterns in the management of Graves’ disease: comparison with European and North American surveys. Eur Thyroid J 5:112–119
Vannucchi G, Campi I, Covelli D, Dazzi D, Currò N, Simonetta S et al (2009) Graves’ orbitopathy activation after radioactive iodine therapy with and without steroid prophylaxis. J Clin Endocrinol Metab 94:3381–3386
Träisk F, Tallstedt L, Abraham-Nordling M, Andersson T, Berg G, Calissendorff J et al (2009) Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab 94:3700–3707
Tallstedt L, Lundell G, Blomgren H, Bring J (1994) Does early administration of thyroxine reduce the development of Graves’ ophthalmopathy after radioiodine treatment? Eur J Endocrinol 130:494–497
Perros P, Kendall-Taylor P, Neoh C, Frewin S, Dickinson J (2005) A prospective study of the effects of radioiodine therapy for hyperthyroidism in patients with minimally active graves’ ophthalmopathy. J Clin Endocrinol Metab 90:5321–5323
Watanabe N, Noh J, Kozaki A, Iwaku K, Sekiya K, Kosuga Y et al (2015) Radioiodine-associated exacerbation of Graves’ orbitopathy in the Japanese population: randomized prospective study. J Clin Endocrinol Metab 100:2700–2708
Gerding M, van der Meer J, Broenink M, Bakker O, Wiersinga W, Prummel M (2000) Association of thyrotropin receptor antibodies with the clinical features of Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 52:267–271
Eckstein A, Plicht M, Lax H, Neuhäuser M, Mann K, Lederbogen S et al (2006) Thyrotropin receptor autoantibodies are independent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of disease. J Clin Endocrinol Metab 91:3464–6470
Marcocci C, Kahaly G, Krassas G, Bartalena L, Prummel M, Stahl M et al (2011) Selenium and the course of mild Graves’ orbitopathy. N Engl J Med 364:1920–1931
Morgenstern K, Evanchan J, Foster J, Cahill K, Burns J, Holck D et al (2004) Botulinum toxin type a for dysthyroid upper eyelid retraction. Ophthalmic Plast Reconstr Surg 20:181–185
Costa P, Saraiva F, Pereira I, Monteiro M, Matayoshi S (2009) Comparative study of Botox injection treatment for upper eyelid retraction with 6-month follow-up in patients with thyroid eye disease in the congestive or fibrotic stage. Eye (London) 23:767–773
Nava Castañeda A, Tovilla Canales J, Garnica Hayashi L, Velasco Y Levy A (2017) Management of upper eyelid retraction associated with dysthyroid orbitopathy during the acute inflammatory phase with botulinum toxin type A. J Fr Ophtthalmol 40:279–284
Marcocci C, Marinò M (2012) Treatment of mild, moderate-to-severe and very severe Graves’ orbitopathy. Best Pract Res Clin Endocrinol Metab 26:325–337
Bhatti M, Dutton J (2014) Thyroid eye disease: therapy in the active phase. Best Pract Res Clin Endocrinol Metab 34:186–197
Kumari R, Chandra Saha B (2018) Advances in the management of thyroid eye disease: an overview. Int Ophthalmol 38:2247–2255
Duntas L, Benvenga S (2015) Selenium: an element for life. Endocrine 48:756–775
Wu Q, Rayman M, Lv H, Schomburg L, Cui B, Gao C et al (2015) Low population selenium status is associated with increased prevalence of thyroid disease. J Clin Endocrinol Metab 100:4037–4047
Vunta H, Davis F, Palempalli U, Bhat D, Arner R, Thompson J et al (2007) The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta 12,14-prostaglandin J2 in macrophages. J Biol Chem 282:17964–17973
Saranac L, Zivanovic S, Bjelakovic B, Stamenkovic H, Novak M, Kamenov B (2011) Why is the thyroid so prone to autoimmune disease? Horm Res Paediatr 75:157–165
Schomburg L (2011) Selenium, selenoproteins and the thyroid gland: interactions in health and disease. Nat Rev Endocrinol 8:160–171
Ventura M, Melo M, Carrilho F (2017) Selenium and thyroid disease: from pathophysiology to treatment. Int J Endocrinol 2017:1297658
Carlson B, Yoo M, Shrimali R, Irons R, Gladyshev V, Hatfield D et al (2010) Role of selenium-containing proteins in T-cell and macrophage function. Proc Nutr Soc 69:300–310
Vrca V, Skerb F, Cepelak I, Romic Z, Mayer L (2004) Supplementation with antioxidants in the treatment of Graves’ disease; the effect on glutathione peroxidase activity and concentration of selenium. Clin Chim Acta 341:55–63
Kahaly G, Riedl M, König J, Diana T, Schomburg L (2017) Double-blind, placebo-controlled, randomized trial of selenium in Graves’hiperthyroidism. J Clin Endocrinol Metab 102:4333–4341
Strianese D (2017) Update of Graves’ disease: advances in treatment of mild, moderate and severe thyroid eye disease. Curr Opin Ophthalmol 28:505–513
Stranges S, Navas-Acien A, Rayman M, Guallar E (2010) Selenium status and cardiometabolic health: state of evidence. Nutr Cardiovasc Dis 20:754–760
Rayman M (2012) Selenium and human health. Lancet 379:12561268
Rocourt C, Cheng W (2013) Selenium supranutrition: are the potential benefits of chemoprevention outweighed by the promotion of diabetes and insulin resistance? Nutrients 5:1349–1365
Heufelder A, Wenzel B, Bahn R (1993) Glucocorticoids modulate the synthesis and expression of a 72 kDa heat shock protein in cultured Graves’ retroocular fibroblast. Acta Endocrinol (Copenh) 128:41–50
Krassas G, Heufelder A (2001) Immunosuppressive therapy in patients with thyroid eye disease: an overview of current concepts. Eur J Endocrinol 144:311–318
Zang S, Ponto K, Kahaly G (2011) Clinical review: intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab 96:320–332
Ayabe R, Rootman D, Hwang C, Ben-Artzi A, Golberg R (2014) Adalimumab as steroid-sparing treatment of inflammatory-stage thyroid eye disease. Ophthal Plast Reconstr Surg 30:415–419
Stiebel-Kalish H, Robenshtok E, Hasanreisoglu M, Ezrachi D, Shimon I, Leibovici L (2009) Treatment modalities for Graves’ ophthalmopathy: systematic review and metaanalysis. J Clin Endocrinol Metab 94:2708–2716
Mou P, Jiang L, Zhang Y, Li Y, Lou H, Zeng C et al (2015) Common immunosuppressive monotherapy for Graves’ ophthalmopathy: a meta-analysis. PLoS ONE 10:e0139544
Kahaly G, Pitz S, Hommel G, Dittmar M (2005) Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab 90:5234–5240
Bartalena L, Pinchera A, Marcocci C (2000) Management of Graves’ ophthalmopathy: reality and perspectives. Endocr Rev 21:168–199
Sisti E, Coco B, Menconi F, Leo M, Rocchi R et al (2015) Intravenous glucocorticoid therapy for Graves’ ophthalmopathy and acute liver damage: an epidemiological study. Eur J Endocrinol 172:269–276
Bartalena L, Krassas G, Wiersinga W, Marcocci C, Salvi M et al (2012) Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopahty. J Clin Endocrinol Metab 97:4454–4463
Covelli D, Vannucchi G, Campi I, Currò N, D’Ambrosio R, Maggioni M et al (2015) Statins may increase the risk of liver dysfunction in patients treated with steroids for active Graves’ orbitopathy. J Clin Endocrinol Metab 100:1731–1737
Sisti E, Menconi F, Leo M, Profilo M, Mautone T, Mazzi B et al (2015) Long-term outcome of Graves’ orbitopathy following high-dose intravenous glucocorticoids and orbital radiotherapy. J Endocrinol Invest 38:661–668
Macchia P, Bagattini M, Lupoli G, Vitale G, Fenzi G (2001) High-dose intravenous corticosteroid therapy for Graves’ ophthalmopathy. J Endocrinol Invest 24:152–158
Smith J, Rosenbaum J (2001) A role methotrexate in the management of non-infectious orbital inflammatory disease. Br J Ophthalmol 85:1200–1224
Emon M, Kodamullil A, Karki R, Younesi E, Hofmann-Apitius M (2017) Using drugs as molecular probes: a computational chemical biology approach in neurodegenerative disease. J Alzheimers Dis 56:677–686
Prummel M, Mourits M, Berghout A, Krenning E, van der Gaag R, Koornneef L et al (1989) Prednisone and cyclosporine in treatment of severe Graves’ ophthalmopathy. N Engl J Med 321:1353–1359
Kahaly G, Schrezenmeir J, Krause U, Schweikert B, Meuer S, Muller W et al (1986) Ciclosporin and prednisone v. prednisone in treatment of Graves’ ophthalmopathy: a controlled, randomized and prospective study. Eur J Clin Invest 16:415–422
Engel P, Gómez-Puerta J, Ramos-Casals M, Lozano F, Bosch X (2011) Therapeutic targeting of B cells for rheumatic autoimmune disease. Pharmacol Rev 63:127–156
Salvi M, Vannucchi G, Currò N, Introna M, Rossi S, Bonara P et al (2012) Small dose of Rituximab for Graves’ orbitopathy: new insights into the mechanism of action. Arch Ophthalmol 130:122–124
Salvi M, Vannucchi G, Beck-Peccoz P (2013) Potential utility of rituximab for Graves’ orbitopathy. J Clin Endocrinol Metab 98:4291–4299
Salvi M, Vannucchi G, Currò N, Campi I, Covell D, Dazzi D et al (2015) Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab 100:422–431
Stan M, Garrity J, Carraza Leon B, Prabin T, Bradley E, Bahn R (2015) Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab 100:432–441
Stan M, Salvi M (2017) Management of endocrine disease: rituximab therapy for Graves’ orbitopathy—lessons from randomized control trials. Eur J Endocrinol 176:101–109
van Vollenhoven R, Emery P, Bingham CO, Keystone E, Fleischmann R, Furst D et al (2013) Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann Rheum Dis 72:1496–1502
Descotes J (2009) Immunotoxicity of monoclonal antibodies. MAbs 1:104–111
Chen H, Shan S, Mester T, Wei Y, Douglas R (2015) TSH-Mediated TNFα production in human fibrocytes is inhibited by teprotumumab, an IGF-1R antagonist. PLoS ONE 10:e0130322
Donahue K, Gartlehner G, Jonas D, Lux L, Thieda P, Jonas B et al (2008) Systematic review: comparative effectiveness and harms of disease-modifying medications for rheumatoid arthritis. Ann Intern Med 148:124–134
Komorowski J, Jankiewicz-Wika J, Siejka A, Lawnicka H, Kłysik A, Goś R et al (2007) Monoclonal anti-TNFalpha antibody (infliximab) in the treatment of patient with thyroid associated ophthalmopathy. Klin Oczna 109:457–460
Paridaens D, van den Bosch W, van der Loos T, Krenning E, van Hagen P (2005) The effect of etanercept on Graves’ ophthalmopathy: a pilot study. Eye (Lond) 19:1286–1289
de-Pablo-Gómez-de-Liaño L, Fernández-Vigo J, Troyano-Rivas J, Niño-Rueda C, Romo-López Á, Gómez de Liaño R (2018) Response to tocilizumab treatment in Graves’ ophthalmopathy by measuring rectus muscle thickness and chemosis using optical coherence tomography. Arch Soc Esp Oftalmol 93:386–391
Pérez-Moreiras J, Alvarez-López A, Gómez E (2014) Treatment of active corticosteroid-resistant graves’ orbitopathy. Plast Reconstr Surg 30:162–167
Pérez-Moreiras J, Gomez-Reino J, Maneiro J, Perez-Pampin E, Romo Lopez A, Rodríguez Alvarez F et al (2018) Efficacy of tocilizumab in patients with moderate to severe corticosteroid resistant graves orbitopathy: a randomized clinical trial. Am J Ophthalmol 195:181–190
Koike T, Harigai M, Inokuma S, Ishiguro N, Ryu J, Takeuchi T et al (2014) Effectiveness and safety of tocilizumab: postmarketing surveillance of 7901 patients with rheumatoid arthritis in Japan. J Rheumatol 41:15–23
Chen H, Mester T, Raychaudhuri N, Kauh C, Gupta S, Smith T et al (2014) Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. J Clin Endocrinol Metab 99:1635–1640
Ye X, Bo X, Hu X, Cui H, Lu B, Shao J et al (2017) Efficacy and safety of mycophenolate mofetil in patients with active moderate-severe Graves’ orbitopathy. Clin Endocrinol (Oxf) 86:247–255
Kahaly G, Riedl M, König J, Pitz S, Ponto K, Diana T et al (2018) Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet Diabetes Endocrinol 6:287–298
Seitz M (1999) Molecular and cellular effects of methotrexate. Curr Opin Rheumatol 11:226–232
Bartalena L, Tanda M, Medea A, Marcocci C, Pinchera A (2002) Novel approaches to the management of graves’ ophthalmopathy. Hormones (Athens) 1:76–90
Sipkova Z, Insull E, David J, Turner H, Keren S, Norris J (2018) Early use of steroid-sparing agents in the inactivation of moderate-to-severe active thyroid eye disease: a step-down approach. Clin Endocrinol (Oxf) 89:834–839
Pasquali D, Vassallo P, Esposito D, Bonavolontà G, Bellastella A, Sinisi A (2000) Somatostatin receptor gene expression and inhibitory effects of octreotide on primary cultures of orbital fibroblasts from Graves’ ophthalmopathy. J Mol Endocrinol 25:63–71
Stan M, Garrity J, Bradley E, Woog J, Bahn M, Brennan M et al (2006) Randomized, double-blind, placebo-controlled trial of long-acting release octreotide for treatment of Graves’ ophthalmopathy. J Clin Endocrinol Metab 91:4817–4824
Tanda L, Bartalena L (2006) Currently available somatostatin analogs are not good for Graves’ orbitopathy. J Endocrinol Invest 29:389–390
Chang T, Liao S (2006) Slow-release lanreotide in Graves’ ophthalmopathy: a double-blind randomized, placebo-controlled clinical trial. J Endocrinol Invest 29:413–422
Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G (2002) SOM 230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and unique antisecretory profile. Eur J Endocrinol 146:707–716
Tahara S, Murakami M, Kaneko T, Shimatsu A (2017) Efficacy and safety of long-acting pasireotide in Japanese patients with acromegaly or pituitary gigantism: results from a multicenter, open-label, randomized, phase 2 study. Endocrinol J 64:735–747
Gerding M, van der Zant F, van Royen E, Koornneef L, Krenning E, Wiersinga W et al (1999) Octreotide-scintigraphy is a disease-activity parameter in Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 50:373–379
Tang F, Chen X, Mao Y, Wan S, Ai S, Yang H et al (2017) Orbital fibroblast of Graves’ orbitopathy stimulated with proinflammatory cytokines promote B cell survival by secreting BAFF. Mol Cell Endocrinol 446:1–11
Shen S, Chan A, Sfikakis P, Hsiu Ling A, Detorakis E, Boboridis K et al (2013) B-cell targeted therapy with rituximab for thyroid eye disease: closer to the clinic. Surv Ophthalmol 58:252–265
McCoy A, Kim D, Gillespie E, Atkins S, Smith T, Douglas R (2014) Rituximab (Rituxan) therapy for severe thyroid-associated ophthalmopathy diminishes IGF-1R (+) T cells. Clin Endocrinol Metab 99:1294–1299
van Vollenhoven R, Wax S, Li Y, Tak P (2015) Safety and efficacy of atacicept in combination with rituximab for reducing the signs and symptoms of rheumatoid arthritis: a phase II, randomized, double-blind, placebo-controlled pilot trial. Arthritis Reumatol 67:2828–2836
Lenert A, Lenert P (2015) Current and emerging treatment options for ANCA-associated vasculitis: potential role of belimumab and other BAFF/APRIL targeting agents. Drug Des Dev Ther 9:333–347
Hewett K, Sanders D, Grove R, Broderick C, Rudo T, Bassiri A et al (2018) Randomized study of adjunctive belimumab in participants with generalized myasthenia gravis. Neurology 90:1425–1434
van Steensel L, Paridaens D, van Meurs M, van Hagen P, van den Bosch W, Kuijpers R et al (2012) Orbit-infiltrating mast cells, monocytes and macrophages produce PDGF isoforms that orchestrate orbital fibroblast activation in Graves’ ophthalmopathy. J Clin Endocrinol Metab 97:400–408
van Steensel L, Paridaens D, Schrijver B, Dingjan G, van Daele P, van Hagen P et al (2009) Imatinib mesylate and AMN107 inhibit PDGF-signaling in orbital fibroblasts: a potential treatment for Graves’ ophthalmopathy. Invest Ophthalmol Vis Sci 50:3091–3098
Kim T, Rea D, Schwarz M, Grille P, Nicolini F, Rosti G et al (2013) Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia 27:1316–1321
Li H, Fitchett C, Kozdon K, Jayaram H, Rose G, Bailly M et al (2014) Independent adipogenic and contractile properties of fibroblasts in Graves’ orbitopathy: an in vitro model for the evaluation of treatments. PLoS ONE 9:e95586
Borriello A, Caldarelli I, Basile M, Bencivenga D, Tramontano A, Perrotta S et al (2011) The tyrosine kinase inhibitor dasatinib induces a marked adipogenic differentiation of human multipotent mesenchymal stromal cells. PLoS ONE 6:e28555
Virakul S, Dalm V, Paridaens D, van den Bosch W, Hirankarn N, van Hagen P et al (2014) The tyrosine kinase inhibitor dasatinib effectively blocks PDGF-induced orbital fibroblast activation. Graefes Arch Clin Exp Ophthalmol 252:1101–1109
Zhang L, Grennan-Jones F, Draman M, Lane C, Morris D, Dayan C et al (2014) Possible targets for nonimmunosuppressive therapy of Graves’ orbitopathy. J Clin Endocrinol Metab 99:1183–1190
Kurtz J, Ray-Coquard I (2012) PI3 kinase inhibitors in the clinic: an update. Anticancer Res 32:2463–2470
Gershengorn M, Neumann S (2012) Update in TSH receptor agonists and antagonists. J Clin Endocrinol Metab 97:4287–4292
Fleischmann R, Schechtman J, Bennett R, Handel M, Burmester G, Tesser J et al (2003) Anakinra, a recombinant human interleukin-1 receptor antagonist (r-metHuIL-1ra), in patients with rheumatoid arthritis: a large, international, multicenter, placebo-controlled trial. Arthritis Rheum 48:927–934
Daifotis A, Koenig S, Chatenoud L, Herold K (2013) Anti-CD3 clinical trials in type 1 diabetes mellitus. Clin Immunol 149:268–278
Rhiu S, Chae M, Lee E, Lee J, Yoon J (2014) Effect of tanshinone IIA in an in vitro model of Graves’ orbitopathy. Invest Ophthalmol Vis Sci 55:5900–5910
Estcourt S, Hickey J, Perros P, Dayan C, Vaidya B (2009) The patient experience of services for thyroid eye disease in the United Kingdom: results of a nationwide survey. Eur J Endocrinol 16:483–487
Acknowledgements
This work has received financial support from ISCIII (RD16/0008/0003) cofounded by European Regional Development Fund (FEDER), and Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia (Centro Singular de Investigación de Galicia Acreditación 2016–2019, ED431G/05).
Funding
This study was funded by ISCIII (RD16/0008/0003) cofounded by European Regional Development Fund (FEDER), and Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia (Centro Singular de Investigación de Galicia Acreditación 2016–2019, ED431G/05).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pouso-Diz, J.M., Abalo-Lojo, J.M. & Gonzalez, F. Thyroid eye disease: current and potential medical management. Int Ophthalmol 40, 1035–1048 (2020). https://doi.org/10.1007/s10792-019-01258-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-019-01258-7