Abstract
Purpose
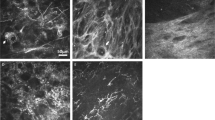
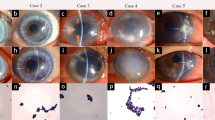
To evaluate the usefulness of serial in vivo confocal microscopy (IVCM) examinations to measure hyphal density for monitoring the treatment success among patients with fungal keratitis, and to compare the hyphal diameter as well as branching angle as a way of differentiation between Aspergillus and Fusarium species observed in IVCM.
Study design
Prospective nonrandomized study.
Patients and methods
The study was conducted from February 2015 to September 2016. Hyphal diameter, density and branching angle measurements were performed using IVCM at admission and on a weekly basis for at least 2 weeks after the start of treatment.
Results
During the period of study, 65 patients with culture-confirmed fungal keratitis were recruited. Of them, 40 were culture-positive for Fusarium spp. and 25 patients for Aspergillus spp. Before the start of treatment, the mean branching angle did not differ between the two species and the mean hyphal diameter was statistically higher for Aspergillus spp. (p = 0.029). Two weeks after the start of treatment, the mean hyphal diameter was statistically lower (p < 0.001) in the treatment failure group. Also the hyphal density significantly decreased with successful treatment (p < 0.05).
Conclusion
Decreasing hyphal density in serial IVCMs might be used as an indicator to predict the successful response of fungal ulcers to treatment. Branching angle is not different between Aspergillus and Fusarium keratitis. The mean hyphal diameter is significantly lower in the treatment failure group.



Similar content being viewed by others
References
Takezawa Y, Shiraishi A, Noda E et al (2010) Effectiveness of in vivo confocal microscopy in detecting filamentous fungi during clinical course of fungal keratitis. Cornea 29(12):1346–1352
Chidambaram JD, Prajna NV, Larke N, Macleod D, Srikanthi P, Lanjewar S (2017) In vivo confocal microscopy appearance of Fusarium and Aspergillus species in fungal keratitis. Br J Ophthalmol 101(8):1119–1123
Iyer SA, Tuli SS, Wagoner RC (2006) Fungal keratitis: emerging trends and treatment outcomes. Eye Contact Lens 32:267–271
Shukla PK, Kumar M, Keshava GB (2008) Mycotic keratitis: an overview of diagnosis and therapy. Mycoses 51(3):183–199
Sun CQ, Lalitha P, Prajna NV et al (2014) Association between in vitro susceptibility to natamycin and voriconazole and clinical outcomes in fungal keratitis. Ophthalmology 121(8):1495–1500
Mahmoudi S, Masoomi A, Ahmadikia K et al (2018) Fungal keratitis: an overview of clinical and laboratory aspects. Mycoses 61(12):916–930. https://doi.org/10.1111/myc.12822
Winchester K, Mathers WD, Sutphin JE (1997) Diagnosis of Aspergillus keratitis in vivo with confocal microscopy. Cornea 16(1):27–31
Avunduk AM, Beuerman RW, Varnell ED, Kaufman HE (2003) Confocal microscopy of Aspergillus fumigatus keratitis. Br J Ophthalmol 87(4):409–410
Brasnu E, Bourcier T, Dupas B et al (2007) In vivo confocal microscopy in fungal keratitis. Br J Ophthalmol 91(5):588–591
Lee S, Yun NR, Kim KH et al (2010) Discrepancy between histology and culture in filamentous fungal infections. Med Mycol 48(6):886–888
Schofield CM, Murray CK, Horvath EE et al (2007) Correlation of culture with histopathology in fungal burn wound colonization and infection. Burns 33(3):341–346
Thomas PA (2003) Current perspectives on ophthalmic mycoses. Clin Microbiol Rev 16(4):730–797
Zbiba W, Baba A, Bouayed E, Abdessalem N, Daldoul A (2016) A 5-year retrospective review of fungal keratitis in the region of Cap Bon. J Fr Ophtalmol 39(10):843–848
Rautaraya B, Sharma S, Kar S, Das S, Sahu SK (2011) Diagnosis and treatment outcome of mycotic keratitis at a tertiary eye care center in eastern India. BMC Ophthalmol 11:39
Punia RS, Kundu R, Chander J, Arya SK, Handa U, Mohan H (2014) Spectrum of fungal keratitis: clinicopathologic study of 44 cases. Int J Ophthalmol 7(1):114–117
Prajna NV, Krishnan T, Mascarenhas J et al (2012) Predictors of outcome in fungal keratitis. Eye 26(9):1226–1231
Ray KJ, Prajna NV, Lalitha P et al (2018) The significance of repeat cultures in the treatment of severe fungal keratitis. Am J Ophthalmol 189:41–46
Acknowledgements
The authors should thank Mrs. Kasiri and Dr. Abedini because of their effort in obtaining confocal sequence imaging and microbiologic assessment, respectively.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tabatabaei, S.A., Soleimani, M., Tabatabaei, S.M. et al. The use of in vivo confocal microscopy to track treatment success in fungal keratitis and to differentiate between Fusarium and Aspergillus keratitis. Int Ophthalmol 40, 483–491 (2020). https://doi.org/10.1007/s10792-019-01209-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-019-01209-2