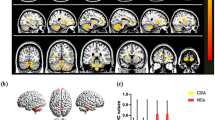
Abstract
Evaluation of brain cluster activation using the functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI) was sought in strabismic amblyopes. In this hospital-based case–control cross-sectional study, fMRI and DTI were conducted in strabismic amblyopes before initiation of any therapy and after visual recovery following the administration of occlusion therapy. FMRI was performed in 10 strabismic amblyopic subjects (baseline group) and in 5 left strabismic amblyopic children post-occlusion therapy after two-line visual improvement. Ten age-matched healthy children with right ocular dominance formed control group. Structural and functional MRI was carried out on 1.5T MR scanner. The visual task consisted of 8 Hz flickering checkerboard with red dot and occasional green dot. Blood-oxygen-level-dependent (BOLD) fMRI was analyzed using statistical parametric mapping and DTI on NordicIce (NordicNeuroLab) softwares. Reduced occipital activation was elicited when viewing with the amblyopic eye in amblyopes. An ‘ipsilateral to viewing eye’ pattern of calcarine BOLD activation was observed in controls and left amblyopes. Activation of cortical areas associated with visual processing differed in relation to the viewing eye. Following visual recovery on occlusion therapy, enhanced activity in bilateral hemispheres in striate as well as extrastriate regions when viewing with either eye was seen. Improvement in visual acuity following occlusion therapy correlates with hemodynamic activity in amblyopes.


Similar content being viewed by others
Abbreviations
- fMRI:
-
Functional magnetic resonance imaging
- DTI:
-
Diffusion tensor imaging
- BOLD:
-
Blood oxygen level dependent
- SPM8:
-
Statistical parametric mapping
- LGN:
-
Lateral geniculate nucleus
- FA:
-
Fractional anisotropy
- EPI:
-
Echo planar imaging
- CBRG:
-
Flickering checkerboard with red dot and occasional green dot
- ANOVA:
-
Analysis of variance
- BA:
-
Brodmann areas
- RE:
-
Right eye
- LE:
-
Left eye
- ROI:
-
Region of interest
References
Flom MC, Neumaier RW (1966) Prevalence of amblyopia. Public Health Rep 81:329–341
Friedman DS, Repka MX, Katz J, Giordano L, Ibironke J, Hawse P, Tielsch JM (2009) Prevalence of amblyopia and strabismus in white and African American children aged 6 through 71 months the Baltimore Pediatric Eye Disease Study. Ophthalmology 116:2128-34.e1-2
Pai AS, Rose KA, Leone JF, Sharbini S, Burlutsky G, Varma R, Wong TY, Mitchell P (2012) Amblyopia prevalence and risk factors in Australian preschool children. Ophthalmology 119:138–144
Algaze A, Roberts CJ, Leguire LE et al (2002) Functional magnetic resonance imaging as a tool for investigating amblyopia in the human visual cortex. J AAPOS 6:300–308
Barnes GR, Hess RF, Dumoulin SO et al (2001) The cortical deficit in humans with strabismic amblyopia. J Physiol 533:281–297
Imamura K, Richter H, Fischer H et al (1997) Reduced activity in the extrastriate visual cortex of individuals with strabismic amblyopia. Neurosci Lett 225:173–176
Li C, Cheng L, Yu Q et al (2012) Relationship of visual cortex function and visual acuity in anisometropic amblyopic children. Int J Med Sci 9:115–120
Hess RF, Thompson B, Gole G, Mullen KT (2009) Deficient responses from the lateral geniculate nucleus in humans with amblyopia. Eur J Neurosci 29:1064–1070
Hess RF, Thompson B, Gole G, Mullen KT (2010) The amblyopic deficit and its relationship to geniculo-cortical processing streams. J Neurophysiol 104:475–483
Choi MY, Lee K, Hwang J et al (2001) Comparison between anisometropic and strabismic amblyopia using functional magnetic resonance imaging. Br J Ophthalmol 85:1052–1056
Anderson SJ, Holliday IE, Harding GF (1999) Assessment of cortical dysfunction in human strabismic amblyopia using magnetoencephalography (MEG). Vision Res 39:1723–1738
Conner IP, Odom JV, Schwartz TL, Mendola JD (2007) Retinotopic maps and foveal suppression in the visual cortex of amblyopic adults. J Physiol 583(Pt 1):159–173
Simons K, Gotzler KC, Vitale S (1997) Penalization versus parttime occlusion and binocular outcome in treatment of strabismicamblyopia. Ophthalmology 104:2156–2160
Harrad R (2000) The efficacy of occlusion for strabismic amblyopia. Can an optimal duration be identified? Br J Ophthalmol 84:561
Rogers GL (2003) Functional magnetic resonance imaging (fMRI) and effects of l-dopa on visual function in normal and amblyopic subjects. Trans Am Ophthalmol Soc 101:401–415
Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed 15:435–455
Andersson JL, Hutton C, Ashburner J et al (2001) Modeling geometric deformations in EPI time series. Neuroimage. 13:903–919
Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009) Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30:2907–2926
Talairach J (1988) Tournoux P in “co-planar stereotaxic atlas of the human brain”. Thieme Medical Publishers, New York
de Marco G, Menuel C, Guillevin R et al (2008) Clinical interest of fMRI and functional exploration methods of brain activity and interactivity: physical and neurophysiological considerations. J Neuroradiol 35:131–143
Fox PT, Raichle ME (1984) Stimulus rate dependence of regional cerebral blood flow in human striate cortex, demonstrated by positron emission tomography. J Neurophysiol 51:1109–1120
Mentis MJ, Alexander GE, Grady CL et al (1997) Frequency variation of a pattern-flash visual stimulus during PET differentially activates brain from striate through frontal cortex. Neuroimage 5:116–128
Tankus A, Fried I (2012) Visuomotor coordination and motor representation by human temporal lobe neurons. J Cogn Neurosci 24:600–610
Kravitz DJ, Saleem KS, Baker CI, Mishkin M (2011) A new neural framework for visuospatial processing. Nat Rev Neurosci 12:217–230
Liu H, Stufflebeam SM, Sepulcre J et al (2009) Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proc Natl Acad Sci USA 106:20499–20503
Le Grand R, Mondloch CJ, Maurer D, Brent HP (2003) Expert face processing requires visual input to the right hemisphere during infancy. Nat Neurosci 6:1108–1112
Hamsher KD (1978) Stereopsis and unilateral brain disease. Invest Ophthalmol Vis Sci 17:336–343
De Renzi E (1986) Prosopagnosia in two patients with CT scan evidence of damage confined to the right hemisphere. Neuropsychologia 24:385–389
Van Kleeck MH (1989) Hemispheric differences in global versus local processing of hierarchical visual stimuli by normal subjects: new data and a meta-analysis of previous studies. Neuropsychologia 27:1165–1178
Sadato N, Okada T, Kubota K, Yonekura Y (2004) Tactile discrimination activates the visual cortex of the recently blind naive to Braille: a functional magnetic resonance imaging study in humans. Neurosci Lett 359:49–52
Everts R, Lidzba K, Wilke M, Kiefer C, Mordasini M, Schroth G, Perrig W, Steinlin M (2009) Strengthening of laterality of verbal and visuospatial functions during childhood and adolescence. Hum Brain Mapp 30:473–483
Crosson B, Moore AB, McGregor KM, Chang YL, Benjamin M, Gopinath K, Sherod ME, Wierenga CE, Peck KK, Briggs RW, Rothi LJ, White KD (2009) Regional changes in word-production laterality after a naming treatment designed to produce a rightward shift in frontal activity. Brain Lang 111:73–85
Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Davidson RJ (2001) Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry 158:405–415
Levy LM, Henkin RI, Lin CS, Hutter A, Schellinger D (1998) Increased brain activation in response to odors in patients with hyposmia after theophylline treatment demonstrated by fMRI. J Comput Assist Tomogr 22:760–770
Fridriksson J, Morrow-Odom L, Moser D, Fridriksson A, Baylis G (2006) Neural recruitment associated with anomia treatment in aphasia. Neuroimage 32:1403–1412
Sundgren PC (2009) Diffusion Tensor Imaging and Tractography: have They Come of Age? J Neuroophthalmol 29:93–95
Song HY, Qi S, Tang HH et al (2010) MR DTI and DTT study on the development of optic radiation in patients with anisometropia amblyopia. Sichuan Da Xue Xue Bao Yi Xue Ban 41:648–651
Xiao JX, Xie S, Ye JT et al (2007) Detection of abnormal visual cortex in children with amblyopia by voxel-based morphometry. Am J Ophthalmol 143:489–493
Barton JJ (2011) Disorders of higher visual processing. Handb Clin Neurol 102:223–261
Myers EH, Hampson M, Vohr B et al (2010) Functional connectivity to a right hemisphere language center in prematurely born adolescents. Neuroimage 51:1445–1452
Skranes J, Lohaugen GC, Martinussen M, Indredavik MS, Dale AM, Haraldseth O, Vangberg TR, Brubakk AM (2009) White matter abnormalities and executive function in children with very low birth weight. NeuroReport 20:263–266
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose they have no potential conflicts of interest.
Ethical approval
The study was approved by the institutional ethics committee and adheres to the tenets of declaration of Helsinki (1964) and its later amendments.
Ethical standards
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants/their parents included in this study.
Rights and permissions
About this article
Cite this article
Gupta, S., Kumaran, S.S., Saxena, R. et al. BOLD fMRI and DTI in strabismic amblyopes following occlusion therapy. Int Ophthalmol 36, 557–568 (2016). https://doi.org/10.1007/s10792-015-0159-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-015-0159-2