Abstract
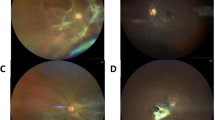
Objectives To describe clinical presentation and results of diagnostic and therapeutic procedures in seven children from an epidemic of panuveitis in the Brazilian Amazonia, as well as environmental analysis and etiological aspects involved. Methods Patients underwent full pediatric and ophthalmic examinations, B-scan, ultrasound biomicroscopy, and serological tests. Ocular samples were thoroughly analyzed, including two enucleation specimens. Environmental investigation encompassed water, soil, and river fauna. Results All patients had bathed in the waters of a regional river, the Araguaia. Six of them presented with intermediate uveitis, with snowbanking. Five had cataract and four showed inferior endothelial opacity, with localized anterior synechiae. One showed total leukoma, with flat anterior chamber. Only two had active uveitis, one of them with anterior chamber nodule. Serology revealed high prevalence of anti-Toxocara canis immunoglobulin G (IgG) antibodies. In three cases, vitreous and lens samples disclosed spicules of freshwater sponges Drulia uruguayensis and D. ctenosclera, also detected in the waters of the river. Conclusion Freshwater sponge spicules could be potential new etiological agents of ocular pathology, but further studies are needed, considering the heterogeneity of the ocular lesions and results of serological and environmental studies.





Similar content being viewed by others
Abbreviations
- BCVA:
-
Best-corrected visual acuity
- ELISA:
-
Enzyme-linked immunosorbent assay
- FBS:
-
Fetal bovine serum
- IOL:
-
Intraocular lens
- KP:
-
Keratic precipitates
- MEM:
-
Minimum Eagle medium
- OD:
-
Right eye
- OS:
-
Left eye
- PAS:
-
Periodic acid Schiff
- PBS:
-
Phosphate buffered saline
- PCR:
-
Polymerase chain reaction
- RFLP:
-
Restriction fragment length polymorphism
- TES:
-
Toxocara excretion-secretion antigen
- UBM:
-
Ultrasound biomicroscopy
References
Magalhães AO, Lemos APF, Cardoso JLC et al (2005) Experimental dermatosis due to cauxi (Drulia uruguayensis Porifera). Mem Inst Butantan 62:118
Magalhães AO, Volkmer-Ribeiro C, Barcellos JFM et al. (2006) Report on two cases of human skin injuries caused by sponge spicules at Amazon. In: Book of abstracts of the 7th international sponge symposium, Búzios, May 7–13th 2006
Volkmer-Ribeiro C, Lenzi HL, Oréfice F et al (2006) Freshwater sponge spicules: a new agent of ocular pathology. Mem Inst Oswaldo Cruz 101:899–903
Kawarabayashi M, Aureliano DP, Raymundo ML et al (2007) Frequency of anti-Toxoplasma gondii antibodies in women attended at public health center network of São Paulo metropolitan region (2001–2005). Rev Inst Adolfo Lutz 66:63–67
Oliveira EJ, Kanamura HY, Lima DM (2005) Efficacy of an enzyme-linked immunosorbent assay as a diagnostic tool for schistosomiasis mansoni in individuals with low worm burden. Mem Inst Oswaldo Cruz 100:421–425. doi:10.1590/S0074-02762005000400013
da Silva RM, Kanamura HY, Camargo ED et al (1998) A comparative study on IgG-ELISA, IgM-IFT and Kato-Katz methods for epidemiological purposes in a low endemic area for schistosomiasis. Mem Inst Oswaldo Cruz 93(Suppl 1):279–282
de Savigny DH, Voller A, Woodruff AW (1979) Toxocariasis: serological diagnosis by enzyme immunoassay. J Clin Pathol 32:284–288. doi:10.1136/jcp.32.3.284
White TJ, Bruns TD, Lee SB (1990) Amplification and direct sequencing of fungal ribossomal RNA genes for phylogenetics. In: Innis NA, Gelfand J, White TJ et al (eds) PCR protocols: a guide of methods and applications. Academic, San Diego, pp 315–322
Nogueira ML, Carvalho AF, Barbosa EF et al (1998) Diagnosis of mucocutaneous herpetic infections by PCR without DNA extraction. Mem Inst Oswaldo Cruz 93:213–214
Marques JT, Trindade GD, Da Fonseca FG et al (2001) Characterization of ATI, TK and IFN-alpha/betaR genes in the genome of the BeAn 58058 virus, a naturally attenuated wild Orthopoxvirus. Virus Genes 23:291–301. doi:10.1023/A:1012521322845
Lennert K (1978) Malignant lymphomas other than Hodgkin’s disease. Springer-Verlag, Berlin, pp 1–71
Bogomoletz W (1980) Avantages de la coloration par le rouge Sirius de l’amyloide et des eosinophiles. Arch Anat Cytol Pathol 28:252–253
Luque EH, Montes GS (1989) Progesterone promotes a massive infiltration of the rat uterine cervix by the eosinophilic polymorphonuclear leukocytes. Anat Rec 223:257–265. doi:10.1002/ar.1092230304
Dolber PC, Spach MS (1993) Conventional and confocal fluorescence microscopy of collagen fibers in the heart. J Histochem Cytochem 41:465–469
Rathinam S, Fritsche TR, Srinivasan M et al (2001) An outbreak of trematode-induced granulomas of the conjunctiva. Ophthalmology 108:1223–1229. doi:10.1016/S0161-6420(01)00604-2
Rathinam SR, Usha KR, Rao NA (2002) Presumed trematode-induced granulomatous anterior uveitis: a newly recognized cause of intraocular inflammation in children from south India. Am J Ophthalmol 133:773–779. doi:10.1016/S0002-9394(02)01435-6
Wallace GD, Rosen L (1969) Techniques for recovering and identifying larvae of Angiostrongylus cantonensis from molluscs. Malacologia 7:427–438
Moraes RG (1948) Contribuição para o estudo do Strongyloides stercoralis e da estrongiloidose no Brasil. Rev Serv Saude Publ 1:507–624
Ruiz De Ybanez MR, Garijo M, Goyena M et al (2000) Improved methods for recovering eggs of Toxocara canis from soil. J Helminthol 74:349–353
Rosenberg KD, Feuer WJ, Davis JL (2004) Ocular complications of pediatric uveitis. Ophthalmology 111:2299–2306. doi:10.1016/j.ophtha.2004.06.014
Narayana KM, Bora A, Biswas J (2003) Patterns of uveitis in children presenting at a tertiary eye care centre in south India. Indian J Ophthalmol 51:129–132
Friling R, Kramer M, Snir M et al (2005) Clinical course and outcome of uveitis in children. J AAPOS 9:379–382. doi:10.1016/j.jaapos.2005.04.005
BenEzra D, Cohen E, Maftzir G (2005) Uveitis in children and adolescents. Br J Ophthalmol 89:444–448. doi:10.1136/bjo.2004.050609
Kump LI, Cervantes-Castaneda RA, Androudi SN et al (2005) Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology 112:1287–1292. doi:10.1016/j.ophtha.2005.01.044
Tran VT, Lumbroso L, LeHoang P et al (1999) Ultrasound biomicroscopy in peripheral retinovitreal toxocariasis. Am J Ophthalmol 127:607–609. doi:10.1016/S0002-9394(98)00403-6
Chieffi PP, Ueda M, Camargo ED et al (1990) Visceral larva migrans: a seroepidemiological survey in five municipalities of Sao Paulo state, Brazil. Rev Inst Med Trop Sao Paulo 32:204–210
Ellis GS Jr, Pakalnis VA, Worley G et al (1986) Toxocara canis infestation. Clinical and epidemiological associations with seropositivity in kindergarten children. Ophthalmology 93:1032–1037
Marmor M, Glickman L, Shofer F et al (1987) Toxocara canis infection of children: epidemiologic and neuropsychologic findings. Am J Public Health 77:554–559
Campos Junior D, Elefant GR, e Silva EO et al (2003) Frequency of seropositivity to Toxocara canis in children of different socioeconomic strata. Rev Soc Bras Med Trop 36:509–513
Good B, Holland CV, Taylor MR et al (2004) Ocular toxocariasis in schoolchildren. Clin Infect Dis 39:173–178. doi:10.1086/421492
Shields JA (1984) Ocular toxocariasis. A review. Surv Ophthalmol 28:361–381. doi:10.1016/0039-6257(84)90242-X
Stewart JM, Cubillan LD, Cunningham ET Jr (2005) Prevalence, clinical features, and causes of vision loss among patients with ocular toxocariasis. Retina 25:1005–1013. doi:10.1097/00006982-200512000-00009
Basak SK, Singhal P, Hazra TK et al (2006) Avian trematode Philophthalmus. Ophthalmology 113:1063 e1061–1062
Lamothe-Argumedo R, Diaz-Camacho SP, Nawa Y (2003) The first human case in Mexico of conjunctivitis caused by the avian parasite, Philophthalmus lacrimosus. J Parasitol 89:183–185. doi:10.1645/0022-3395(2003)089[0183:TFHCIM]2.0.CO;2
Oréfice F, Simal CJ, Pittella JE (1985) Schistosomotic choroiditis. I. Funduscopic changes and differential diagnosis. Br J Ophthalmol 69:294–299. doi:10.1136/bjo.69.4.294
Pittella JE, Oréfice F (1985) Schistosomotic choroiditis. II. Report of first case. Br J Ophthalmol 69:300–302. doi:10.1136/bjo.69.4.300
Acknowledgements
The authors thank Dr. Marco Antônio Tanure for help in anterior segment photography and surgery, Dr. Valênio Perez França for oculoplastic surgery, Dr. Breno Lino and Dr. Célia Andrade for echography and ultrasound biomicroscopy, respectively, and Christiane Goveia for technical support. The authors are also grateful to the Laboratory of Virology of Instituto de Ciências Biológicas da UFMG and to Instituto Adolpho Lutz. Dr. Fernando Oréfice had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This work was supported by Universidade Federal de Minas Gerais (UFMG), Fundação Oswaldo Cruz (FIOCRUZ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Secretaria de Saúde de Tocantins and Ministério da Saúde – Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vasconcelos-Santos, D.V., Oréfice, F., Fonseca, C.F. et al. Epidemic of unilateral panuveitis in children from Brazilian Amazonia: clinical and etiological aspects in seven patients. Int Ophthalmol 30, 113–125 (2010). https://doi.org/10.1007/s10792-009-9294-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-009-9294-y