Abstract
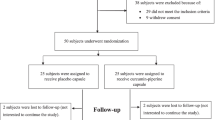
Licorice extract (glycyrrhizin), a potent antiviral, anti-inflammatory, and antioxidant remedy, is a potential therapeutic option for COVID-19. We evaluated the efficacy and safety of licorice in patients with moderate COVID-19. In this study, 60 patients with confirmed COVID-19 were randomly assigned in a 1:1 ratio to receive licorice (at a dose of 760 mg three times a day for seven days) or control groups. The primary outcomes were SPO2, body temperature, and respiratory rate (RR) after the end of the intervention. The findings indicated that SPO2, body temperature, and RR had no significant difference between the groups at the end of the intervention. However, CRP and ALT improved in the licorice group toward the baseline. The number of patients with worse prognoses, LOS, mortality, and the incidence of adverse events were not different between the groups at the end of the study. Licorice had no beneficial effect on the clinical symptoms of COVID-19. Moreover, this intervention demonstrated a safe profile of adverse events. The confirmation of the results of this preparatory trial requires more detailed multiple-center trials with a larger sample size.

Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. Other data requests will be considered by the management group upon written request to the corresponding authors.
References
Adapa S, Chenna A, Balla M, Merugu GP, Koduri NM, Daggubati SR, Gayam V, Naramala S, Konala VM (2020) Covid-19 pandemic causing acute kidney injury and impact on patients with chronic kidney disease and renal transplantation. J Clin Med Res 12:352–361
Alexyuk PG, Bogoyavlenskiy AP, Alexyuk MS, Turmagambetova AS, Zaitseva IA, Omirtaeva ES, Berezin VE (2019) Adjuvant activity of multimolecular complexes based on Glycyrrhiza glabra saponins, lipids, and influenza virus glycoproteins. Arch Virol 164:1793–1803
Amaryan G, Astvatsatryan V, Gabrielyan E, Panossian A, Panosyan V, Wikman G (2003) Double-blind, placebo-controlled, randomized, pilot clinical trial of immunoguard—a standardized fixed combination of Andrographis paniculata nees, with Eleutherococcus senticosus maxim, Schizandra chinensis bail. And Glycyrrhiza glabra L. extracts in patients with familial Mediterranean fever. Phytomedicine 10:271–285
Baloch S, Baloch MA, Zheng T, Pei X (2020) The coronavirus disease 2019 (Covid-19) pandemic. Tohoku J Exp Med 250:271–278
Cavezzi A, Troiani E, Corrao S (2020) Covid-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract 10:1271
Chatow L, Nudel A, Nesher I, Hayo Hemo D, Rozenberg P, Voropaev H, Winkler I, Levy R, Kerem Z, Yaniv Z, Eyal N (2021) In vitro evaluation of the activity of terpenes and cannabidiol against human coronavirus E229. Life (Basel). https://doi.org/10.3390/life11040290
Choi HW, Tian M, Manohar M, Harraz MM, Park SW, Schroeder FC, Snyder SH, Klessig DF (2015) Human gapdh is a target of Aspirin’s primary metabolite salicylic acid and its derivatives. PLoS One 10:E0143447
Cook B, Mascolo M, Bass G, Duffy ME, Zehring B, Beasley T (2022) Has covid-19 complicated eating disorder treatment? An examination of comorbidities and treatment response before and during the covid-19 pandemic. Prim Care Companion Cns Disord. https://doi.org/10.4088/PCC.21m03087
Deore SL, Jaju PS, Baviskar BA (2014) Simultaneous estimation of four antitussive components from herbal cough syrup by hptlc. Int Sch Res Notices 2014:976264
Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, Poucke SV, Liu WY, Zheng MH (2020) Covid-19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol 8:18–24
Fiore C, Eisenhut M, Krausse R, Ragazzi E, Pellati D, Armanini D, Bielenberg J (2008) Antiviral effects of glycyrrhiza species. Phytother Res Ptr 22:141–148
Gao R, Zhang Y, Kang Y, Xu W, Jiang L, Guo T, Huan C (2020) Glycyrrhizin inhibits pedv infection and proinflammatory cytokine secretion via the Hmgb1/Tlr4-Mapk P38 pathway. Int J Mol Sci. https://doi.org/10.3390/ijms21082961
Gomaa AA, Mohamed HS, Abd-Ellatief RB, Gomaa MA, Hammam DS (2022) Advancing combination treatment with glycyrrhizin and boswellic acids for hospitalized patients with moderate covid-19 infection: a randomized clinical trial. Inflammopharmacology 30:477–486
Hoever G, Baltina L, Michaelis M, Kondratenko R, Baltina L, Tolstikov GA, Doerr HW, Cinatl J Jr (2005) Antiviral activity of glycyrrhizic acid derivatives against sars-coronavirus. J Med Chem 48:1256–1259
Ipatova OM, Nasonov EL, Korotaeva TV, Firsov NN, Ivkina OA, Torkhovskaia TI, Archakov AI (2003) Hemorheological and clinical efficiency of a new phospholipid hepatoprotective drug phosphogliv in patients with psoriatic arthritis. Biomed Khim 49:484–490
Kandeel M, Kitade Y, Almubarak A (2020) Repurposing Fda-approved phytomedicines, natural products, antivirals and cell protectives against Sars-COv-2 (Covid-19) Rna-dependent Rna polymerase. PeerJ 8:E10480
Kapadia GJ, Azuine MA, Tokuda H, Hang E, Mukainaka T, Nishino H, Sridhar R (2002) Inhibitory effect of herbal remedies on 12-O-tetradecanoylphorbol-13-acetate-promoted epstein-barr virus early antigen activation. Pharmacol Res 45:213–220
Kim S-H, Hong J-H, Yang W-K, Geum J-H, Kim H-R, Choi S-Y, Kang Y-M, An H-J, Lee Y-C (2020) Herbal combinational medication of Glycyrrhiza glabra, Agastache rugosa containing glycyrrhizic acid, tilianin inhibits neutrophilic lung inflammation by affecting Cxcl 2, interleukin-17/STAT3 signal pathways in a murine model of copd. Nutrients 12:926
Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C (2020) Cardiac and arrhythmic complications in patients with covid-19. J Cardiovasc Electrophysiol 31:1003–1008
Kusum M, Klinbuayaem V, Bunjob M, Sangkitporn S (2004) Preliminary efficacy and safety of oral suspension Sh, combination of five Chinese medicinal herbs, in people living with Hiv/Aids; the phase I/Ii study. J Med Assoc Thai 87:1065–1070
Lan XF, Olaleye OE, Lu JL, Yang W, Du FF, Yang JL, Cheng C, Shi YH, Wang FQ, Zeng XS, Tian NN, Liao PW, Yu X, Xu F, Li YF, Wang HT, Zhang NX, Jia WW, Li C (2021) Pharmacokinetics-based identification of pseudoaldosterogenic compounds originating from glycyrrhiza uralensis roots (Gancao) after dosing lianhuaqingwen capsule. Acta Pharmacol Sin 42:2155–2172
Lee M, Son M, Ryu E, Shin YS, Kim JG, Kang BW, Cho H, Kang H (2015) Quercetin-induced apoptosis prevents Ebv infection. Oncotarget 6:12603–12624
Li H, Hu Y, Tang H, Li S, Ding H, Zhai S, Zhao R (2020a) The potential of glycyrrhizinate in the management of covid-19: a systematic review of the efficacy and safety of glycyrrhizin preparations in the treatment of Sars and Mers. Am J Chin Med 48:1539–1552
Li H, Xue Q, Xu X (2020b) Involvement of the nervous system in sars-cov-2 infection. Neurotox Res 38:1–7
Li J, Xu D, Wang L, Zhang M, Zhang G, Li E, He S (2021) Glycyrrhizic acid inhibits sars-cov-2 infection by blocking spike protein-mediated cell attachment. Molecules. https://doi.org/10.3390/molecules26206090
Manns MP, Wedemeyer H, Singer A, Khomutjanskaja N, Dienes HP, Roskams T, Goldin R, Hehnke U, Inoue H (2012) Glycyrrhizin In patients who failed previous interferon alpha-based therapies: biochemical and histological effects after 52 weeks. J Viral Hepat 19:537–546
Mathew SM, Benslimane F, Althani AA, Yassine HM (2021) Identification of potential natural inhibitors of the receptor-binding domain of the sars-cov-2 spike protein using a computational docking approach. Qatar Med J 2021:12
Miyakawa K, Matsunaga S, Yamaoka Y, Dairaku M, Fukano K, Kimura H, Chimuro T, Nishitsuji H, Watashi K, Shimotohno K, Wakita T, Ryo A (2018) Development of a cell-based assay to identify hepatitis b virus entry inhibitors targeting the sodium taurocholate cotransporting polypeptide. Oncotarget 9:23681–23694
Nabeshima S, Kashiwagi K, Ajisaka K, Masui S, Takeoka H, Ikematsu H, Kashiwagi S (2012) A randomized, controlled trial comparing traditional herbal medicine and neuraminidase inhibitors in the treatment of seasonal influenza. J Infect Chemother 18:534–543
Narkhede RR, Pise AV, Cheke RS, Shinde SD (2020) Recognition of natural products as potential inhibitors of covid-19 main protease (Mpro): in-silico evidences. Nat Prod Bioprospect 10:297–306
Oesch F, Oesch-Bartlomowicz B, Efferth T (2021) Toxicity as prime selection criterion among sars-active herbal medications. Phytomedicine 85:153476
Orlent H, Hansen BE, Willems M, Brouwer JT, Huber R, Kullak-Ublick GA, Gerken G, Zeuzem S, Nevens F, Tielemans WC, Zondervan PE, Lagging M, Westin J, Schalm SW (2006) Biochemical and histological effects of 26 weeks of glycyrrhizin treatment in chronic hepatitis C: a randomized phase ii trial. J Hepatol 45:539–546
Pandit S, Ponnusankar S, Bandyopadhyay A, Ota S, Mukherjee PK (2011) Exploring the possible metabolism mediated interaction of Glycyrrhiza glabra extract with Cyp3a4 and Cyp2d6. Phytother Res 25:1429–1434
Ren CA, Li YX, Cui JY, Sheng ZX, Ran XH, Wang BH, Zhang MH (2013) Efficacy of glycyrrhizin combined with cyclosporine in the treatment of non-severe aplastic anemia. Chin Med J (Engl) 126:2083–2086
Soleymani S, Naghizadeh A, Karimi M, Zarei A, Mardi R, Kordafshari G, Esmaealzadeh N, Zargaran A (2022) Covid-19: general strategies for herbal therapies. J Evid Based Integr Med. https://doi.org/10.1177/2515690X211053641
Tay MZ, Poh CM, Rénia L, Macary PA, Ng LFP (2020) The trinity of covid-19: immunity, inflammation and intervention. Nat Rev Immunol 20:363–374
Tian YM, Dou LP, Yao S, Yao ZL, Zhang QF, Yu L, Jing Y (2013) Protective effect of ademetionine 1, 4-butanedisulfonate on liver injury caused by chemotherapeutic agents. Zhongguo Shi Yan Xue Ye Xue Za Zhi 21:1305–1308
Tolah AM, Altayeb LM, Alandijany TA, Dwivedi VD, El-Kafrawy SA, Azhar EI (2021) Computational and in vitro experimental investigations reveal anti-viral activity of licorice and glycyrrhizin against severe acute respiratory syndrome coronavirus 2. Pharmaceuticals (Basel). https://doi.org/10.3390/ph14121216
Umeoguaju FU, Ephraim-Emmanuel BC, Patrick-Iwuanyanwu KC, Zelikoff JT, Orisakwe OE (2021) Plant-derived food grade substances (Pdfgs) active against respiratory viruses: a systematic review of non-clinical studies. Front Nutr 8:606782
Wang L, Yang R, Yuan B, Liu Y, Liu C (2015) The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm Sin B 5:310–315
Yanagawa Y, Ogura M, Fujimoto E, Shono S, Okuda E (2004) Effects and cost of glycyrrhizin in the treatment of upper respiratory tract infections in members of the Japanese maritime self-defense force: preliminary report of a prospective, randomized, double-blind, controlled, parallel-group, alternate-day treatment assignment clinical trial. Curr Ther Res Clin Exp 65:26–33
Yang R, Yuan BC, Ma YS, Zhou S, Liu Y (2017) The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm Biol 55:5–18
Yu Z, Ohtaki Y, Kai K, Sasano T, Shimauchi H, Yokochi T, Takada H, Sugawara S, Kumagai K, Endo Y (2005) Critical roles of platelets in lipopolysaccharide-induced lethality: effects of glycyrrhizin and possible strategy for acute respiratory distress syndrome. Int Immunopharmacol 5:571–580
Zhang QH, Huang HZ, Qiu M, Wu ZF, Xin ZC, Cai XF, Shang Q, Lin JZ, Zhang DK, Han L (2021) Traditional uses, pharmacological effects, and molecular mechanisms of licorice in potential therapy Of Covid-19. Front Pharmacol 12:719758
Acknowledgements
The authors appreciably thank the trial patients and their families, whose help and participation made this study possible. They would also like to thank the assistance of Irandarouk Pharmaceutical Company for preparing the D-Reglis®tablets and Amin Chemical and Pharmaceutical Company for preparing the hydroxychloroquine sulfate tablets. The Irandarouk Pharmaceutical Company and Amin Chemical and Pharmaceutical Company played no part in the design of the trial the intervention procedures, collection, evaluation, and analysis of data.
Funding
This research was funded by the Hormozgan University of Medical Sciences (no; 990061).
Author information
Authors and Affiliations
Contributions
AA, MF, and MF were responsible for conceptualization, methodology, and software. MF, HD, and SH performed data curation and writing––original draft preparation. PD, AK, and OS were involved in visualization and investigation. AA did supervision. SH were responsible for software and validation. MF was involved in writing––reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
Ethical approval was obtained from the ethics committee of Hormozgan University of Medical Sciences (IR.HUMS.REC.1399.066). This study was also undertaken in accordance with the guidelines of the Declaration of Helsinki and the principles of the International Conference on Harmonization Good Clinical Practice.
Consent for publication
Written informed consent was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ameri, A., Farashahinejad, M., Davoodian, P. et al. Efficacy and safety of licorice (Glycyrrhiza glabra) in moderately ill patients with COVID-19: a randomized controlled trial. Inflammopharmacol 31, 3037–3045 (2023). https://doi.org/10.1007/s10787-023-01352-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01352-4