Abstract
Background
Osthole is a bioactive component reported in medicinal plants such as Angelica pubescens and Cnidium monnieri, known for analgesic activity. However, the toxicity, median effective dose (ED50), and dual modulation of nitric oxide and cyclooxygenase pathways along with inflammatory cytokines of osthole are yet to be determined.
Methods
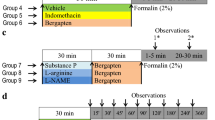
The animals (mice) were assessed for general behaviour and mortality in varying doses (50, 300, and 2000 mg kg−1) of osthole for acute toxicity over 14 days. The analgesic activity was investigated using acetic acid and formalin-induced hyperalgesia, and anti-inflammatory activity was explored in carrageenan-induced paw oedema. ED50 of osthole was calculated using Design Expert software. Involvement of nitric oxide and cyclooxygenase pathways was investigated by agonist challenges with l-arginine and substance P, respectively. The expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) was determined in spinal sections by immunohistochemical analysis. Lipopolysaccharide (LPS) challenge was used to assess in vivo effect on inflammatory cytokines (TNFα and IL-6).
Results
Acute toxicity studies revealed no behavioural abnormality or mortality on osthole treatment and unremarkable histological findings. Osthole was found to significantly decrease acetic acid and formalin-induced hyperalgesia (ED50 = 5.43 mg kg−1) and carrageenan-induced paw oedema with no toxicity symptoms. Osthole produced a marked decrease in iNOS and COX-2 expression as well as TNFα and IL-6. The findings corroborate to modulation of iNOS and COX-2 and inflammatory cytokines by osthole. This study provides promising insights and prospects for application of osthole in pain management.







Similar content being viewed by others
References
Anbar M, Gratt BM (1997) Role of nitric oxide in the physiopathology of pain. J Pain Symptom Manag 14:225–254
Andrade SF, Cardoso LGV, Carvalho JCT, Bastos JK (2007) Anti-inflammatory and antinociceptive activities of extract, fractions and populnoic acid from bark wood of austroplenckiapopulnea. J Ethnopharmacol 109:464–471
Basbaum AL, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanisms of pain. Cell 139:267–284
Bhat AS, Tandan SK, Kumar D, Krishna V, Prakash VR (2008) The interaction between inhibitors of nitric oxide synthase and cyclooxygenase in formalin-induced pain in mice: an isobolographic study. Anesth Analg 106:978–984
Bonassoli VT, Contardi EB, Milani H, Oliveira RMWD (2013) Effects of nitric oxide synthase inhibition in the dorsolateral periaqueductal gray matter on ethanol withdrawal-induced anxiety-like behavior in rats. Psycopharmacology 228:487–498
Bushnell MC, Case LK, Ceko M, Cotton VA, Gracely JL, Low LA, Pitcher MH, Villemure C (2015) Effect of environment on the long-term consequence of chronic pain. J Pain 15:S42–S49
Carballo-Villalobos AI, Gonza’lez-Trujano ME, Alvarado-Va’zquez N, Lopez-Munoz FJ (2017) Pro-inflammatory cytokines involvement in the hesperidin antihyperalgesic effects at peripheral and central levels in a neuropathic pain model. Inflammopharmacology 25:265–269
Cashman JN (1996) The mechanisms of action of NSAIDs in analgesia. Drugs 52:13–23
Cha JH, Kim WK, Ha AW, Kim MH, Chang MJ (2017) Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr Res Pract 11:90–96
Crofford LJ (2010) Adverse effects of chronic opioid therapy for chronic musculoskeletal pain. Nat Rev Rheumatol 6:191–197
Cury Y, Picolo G, Gutierrez VP, Ferreira SH (2011) The dual effect of nitric oxide in the nociceptive system. Nitric Oxide 25:243–254
Duarte IDG, Nakamura M, Ferreira SH (1988) Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz J Med Biol Res 21:341–343
Farrar JT (2010) Advances in clinical research methodology for pain clinical trials. Nat Med 16:1248–1293
Goudet C, Chapuy E, Alloui A, Acher F, Pin JP, Eschalier A (2008) Group III metabotropic glutamate receptors inhibit hyperalgesia in animal models of inflammation and neuropathic pain. Pain 137:112–124
Hajhashemi V, Sajjadi SE, Zomorodkia M (2011) Antinociceptive and anti-inflammatory activities of Bunium persicum essential oil, hydroalcoholic and polyphenolic extracts in animal models. Pharm Biol 49:146–151
Hunskaar S, Hole K (1987) The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 30:103–114
Ibana H, Yoshigai E, Okuyama T, Murakoshi M, Sugiyama K, Nishino H, Nishizawa M (2015) Antipyretic analgesic drugs have different mechanisms for regulation of the expression of inducible nitric oxide synthase in hepatocytes and macrophages. Nitric Oxide 44:61–70
Janes K, Neumann WL, Salyemini D (2012) Anti-superoxide and anti-peroxynitrite strategies in pain suppression. Biochim Biophys Acta 1822:815–821
Ji HJ, Hu JF, Wang YH, Chen XY, Zhou R, Chen NH (2010) Osthole improves chronic cerebral hypoperfusion induced cognitive deficits and neuronal damage in hippocampus. Eur J Pharmacol 636:96–101
Liu J, Hang W, Zhou L, Wang X, Lian Q (2005) Anti-inflammatory effect and mechanism of osthole in rats. J Chin Med Mater 28:1002–1006
Kawabata A, Manabe S, Manabe Y, Takagi H (1994) Effect of topical administration of l-arginine on formalin-induced nociception in the mouse: a dual role of peripherally formed NO in pain modulation. Br J Pharmacol 112:547–550
Koon HW, Zhao D, Zhan Y, Rhee SH, Moyer MP, Pothoulakis C (2006) Substance P stimulates cyclooxygenase-2 and prostaglandin E2 expression through JAK-STAT activation in human colonic epithelial cells. J Immunol 176:5050–5059
Kulkarni SK (2005) Handbook of experimental Pharmacology, 3rd edn. Vallabh Prakashan, New Delhi, p 127
Lam HH, Hanley DF, Trapp BD, Saito S, Raja S, Dawson TM, Yamaguchi H (1996) Induction of spinal cord neuronal nitric oxide synthase (NOS) after formalin injection in the rat hind paw. Neurosci Lett 210:201–204
Li XX, Hara I, Matsumiya T (2002) Effects of osthole on postmenopausal osteoporosis using ovariectomized rats; comparison to the effects of estradiol. Biol Pharm Bull 25:738–742
Liao PC, Chien SC, Ho CL, Wang EIC, Lee SI, Kuo YH, Jeyashoke N, Chen J, Dong WC, Chao LK, Hua KF (2010) Osthole regulates inflammatory mediator expression through modulating NF-kB, mitogen-activated protein kinases, protein kinase C, and reactive oxygen species. J Agric Food Chem 58:10445–10451
Liu J, Xu R, Zhao X (2016) Mechanisms for effect of osthole on inhibiting the growth and invasion of bladder cancer cells. Eur PMC 41:345–352
Machado GC, Maher CG, Ferreira PH, Day RO, Pinheiro MB, Ferreira ML (2016) Non-steroidal anti-inflammatory drugs for spinal pain: a systematic review and meta-analysis. Ann Rheum Dis 76:210597
Martin SL, Power A, Boyle Y, Anderson IM, Silverdale MA, Jones AK (2017) 5-HT modulation of pain perception in humans. Psychopharmacology 234(19):2929–2939
McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM (2007) TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci 104:13525–13530
OECD Guideline for testing of chemicals (2001) Guideline 423: acute oral toxicity- acute toxic class method. Organization for Economic Cooperation and Development, Paris
Ritter J, Flower RJ, Henderson G, Rang H (2011) Nitric oxide: Chemical mediators. Rang and Dale’s Pharmacology, 7th edn. Elsevier Health Sciences, London, United Kingdom, pp 237–243
Smith WL, Murphy RC (2002) The eicosanoids: cyclooxygenase, lipoxygenase and epoxygenase pathways. New Compr Biochem 36:341–371
Tadiwos Y, Nedi T, Engidawork E (2017) Analgesic and anti-inflammatory activities of 80% methanol root extract of Jasminum abyssinicum hochst. ex. Dc. (Oleaceae) in mice. J Ethnopharmacol 202:281–289
Wandji BA, Bomba FDT, Nkeng-Efouet PA, Piegang BN, Kamanyi A, Nguelefack TB (2018) Antihyperalgesic activity of the aqueous and methanol extracts of the leaves of Pittosporum mannii Hook on CFA-induced persistent inflammatory pain. Inflammopharmacology 26:197–205
Wikel S (2013) Ticks and tick-borne pathogens at the cutaneous interface: host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Front Microbiol 19:337
Winter CA, Risely EA, Nuss GW (1962) Carrageenan induced edema in hind paw of the rat as assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 11:544–547
Yamato K, Kataoka T, Nishiyama Y, Taguchi T, Yamaoka K (2013) Antinociceptive effects of radon inhalation on formalin-induced inflammatory pain in mice. Inflammation 36:355–363
Yang SM, Chan YL, Hua KF, Chang JM, Chen HL, Tsai YJ, Chao LK, Feng-Ling Y, Tsai YL, Wu SH, Wang YF, Tsai CL, Chen A, Ka SM (2014) Osthole improves an accelerated focal segmental glomerulosclerosis model in the early stage by activating the Nrf2 antioxidant pathway and subsequently inhibiting NF-κB-mediated COX-2 expression and apoptosis. Free Radic Biol Med 73:260–269
Yang D, Gu T, Wang T, Tang Q, Ma C (2010) Effects of osthole on migration and invasion in breast cancer cells. Biosci Biotechnol Biochem 74:1430–1434
Yoon YW, Sung B, Chung JM (1998) Nitric oxide mediates behavioral signs of neuropathic pain in an experimental rat model. NeuroReport 9:367–372
Zhang ZR, Leung WN, Cheung HY, Chan CW (2015) Osthole: a review on its bioactivities, pharmacological properties, and potential as alternative medicine. Evid Based Complement Altern Med 2015:919616
Acknowledgements
The authors are grateful to the Department of Science and Technology, Government of India, for funding received under EMR (EMR/2016/005878). University Grants Commission, New Delhi, is acknowledged for the financial assistance to Gurjit Singh (UGC-NFSC) and grant under university with potential for Excellence to Guru Nanak Dev University. Statistical analysis performed by Dr. M.S. Bhatti, Associate Professor, Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Singh, G., Bhatti, R., Mannan, R. et al. Osthole ameliorates neurogenic and inflammatory hyperalgesia by modulation of iNOS, COX-2, and inflammatory cytokines in mice. Inflammopharmacol 27, 949–960 (2019). https://doi.org/10.1007/s10787-018-0486-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-018-0486-9