Abstract
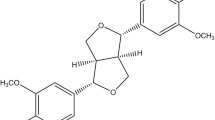
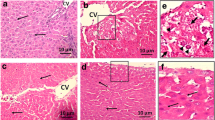
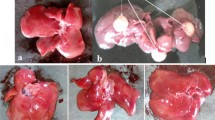
The aerial part of Wedelia calendulacea have been used in Ayurveda, Unani, Tibetan, Siddha and other folk medicine systems to protect the liver and renal tissue. Liver is considered as primary metabolizing site of body, which is prone to damage by endogenous and exogenous toxicants. A reason for liver toxicity, and major causes of the hepatocellular carcinoma (HCC). 19-α-Hydroxyurs-12(13)-ene-28 oic acid-3-O-β-d-glucopyranoside (HEG), a triterpenoids found in the higher plants, has been known to possess protective effect against various toxicants. The aim of the current study was to scrutinize the hepatoprotective mechanism of HEG against DEN-induced oxidative stress, hyperproliferation, inflammation and apoptosis tissue injury in Wistar rats. Invitro cell lines study of HEG scrutinized against the Hep-G2 and HuH-7 cells. A single dose of DEN (200 mg/kg) and double dose of phenobarbitol were administered to induce the liver damage in rats; the dose treatment of HEG was terminated at the end of 22 weeks. Macroscopical study was performed for the confirmation of hepatic nodules. The serum and hepatic samples were collected for further biochemical and histopathological analysis. Hepatic; non-hepatic; Phase I and II antioxidant enzymes were also examined. Additionally, we also scrutinized the inflammatory cytokines viz., tumor necrosis factor-α, interlukin-6, interlukin-1β, and Nuclear factor kappa beta (NF-kB), respectively. Histopathological study was also performed for analyzing the changes during the HCC. HEG confirmed the reduction of growth and deoxyribonucleic acid synthesis of both cell lines. DEN successfully induced the HCC in all group, which was significantly (p < 0.001) altered by the HEG in a dose-dependent manner. The decreased level of pro-inflammatory cytokines and altered membrane-bound enzyme activity were also observed. HEG inhibits the phase I, II and antioxidant enzymes at the effective dose-dependent manner, which were considered as the precursor of the HCC. The alteration of phase I, II and antioxidant enzymes confirmed the inhibition of inflammatory reaction and oxidative stress, which directly or indirectly inhibited the NF-kB expression. Collectively, we can conclude that the HEG inhibited the growth of Hepatocellular carcinoma via attenuating the NF-kB pathway.









Similar content being viewed by others
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- HEG:
-
19-α-Hydroxyurs-12(13)-ene-28 oic acid-3-O-α-d-glucopyranoside
- DEN:
-
Diethylnitrosamine
- TNF-α:
-
Tumor necrosis factor-α
- IL-6:
-
Interlukin-6
- IL-1 β:
-
Interlukin-1β
- NF-kB:
-
Nuclear factor kappa beta
- DNA:
-
Deoxyribonucleic acid
- CYP2E1:
-
Cytochrome P450
- OH:
-
Hydroxyl
- RO2 :
-
Peroxyl
- O2 − :
-
Superoxide
- ONOO− :
-
Peroxynitrite
- H2O2 :
-
Hydrogen peroxide
- ROS:
-
Reactive oxygen species
- AFP:
-
Alpha feto protein
- ALP:
-
Alkaline phosphatase
- AST:
-
Aspartate aminotransferase
- LPO:
-
Lipid peroxidation
- GR:
-
Glutathione reductase
- CAT:
-
Catalase
- GPx:
-
Glutathione peroxidase
- GSH:
-
Glutathione
- SOD:
-
Superoxide dismutase
- GST:
-
Glutathione-S-transferase
- MPO:
-
Myeloperoxidase
- NC:
-
Normal control
References
Agren R, Mardinoglu A, Asplund A et al (2014) Identification of anticancer drugs for hepatocellular carcinoma through personalized genome-scale metabolic modeling. Mol Syst Biol. doi:10.1002/msb.145122
Anwar F, Al-Abbasi FA, Bhatt PC et al (2015a) Umbelliferone β-d-galactopyranoside inhibits chemically induced renal carcinogenesis via alteration of oxidative stress, hyperproliferation and inflammation: possible role of NF-κB. Toxicol Res (Camb) 4:1308–1323. doi:10.1039/c5tx00146c
Anwar F, Mushtaq G, Kazmi I et al (2015b) Anticancer effect of rosiglitazone in rats treated with N-nitrosodiethylamine via inhibition of DNA synthesis: an implication for hepatocellular carcinoma. RSC Adv 5:68385–68391. doi:10.1039/C5RA07291C
Bingül I, Başaran-Küçükgergin C, Tekkeşin MS et al (2013) Effect of blueberry pretreatment on diethylnitrosamine-induced oxidative stress and liver injury in rats. Environ Toxicol Pharmacol 36:529–538. doi:10.1016/j.etap.2013.05.014
Bishayee A, Barnes KF, Bhatia D et al (2010) Resveratrol suppresses oxidative stress and inflammatory response in diethylnitrosamine-initiated rat hepatocarcinogenesis. Cancer Prev Res 3:753–763. doi:10.1158/1940-6207.CAPR-09-0171
Bishayee A, Mbimba T, Thoppil RJ et al (2011) Anthocyanin-rich black currant (Ribes nigrum L.) extract affords chemoprevention against diethylnitrosamine-induced hepatocellular carcinogenesis in rats. J Nutr Biochem 22:1035–1046. doi:10.1016/j.jnutbio.2010.09.001
Butler MS (2008) Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep 25:475–516. doi:10.1039/b514294f
Chen B, Ning M, Yang G (2012) Effect of paeonol on antioxidant and immune regulatory activity in hepatocellular carcinoma rats. Molecules 17:4672–4683. doi:10.3390/molecules17044672
Cho SY, Park SJ, Kwon MJ et al (2003) Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-κB pathway in lipopolysaccharide-stimulated macrophage. Mol Cell Biochem 243:153–160. doi:10.1023/A:1021624520740
Dhanasekaran M, Baskar AA, Ignacimuthu S et al (2009) Chemopreventive potential of Epoxy clerodane diterpene from Tinospora cordifolia against diethylnitrosamine-induced hepatocellular carcinoma. Invest New Drugs 27:347–355. doi:10.1007/s10637-008-9181-9
Ghosh D, Choudhury ST, Ghosh S et al (2012) Nanocapsulated curcumin: oral chemopreventive formulation against diethylnitrosamine induced hepatocellular carcinoma in rat. Chem Biol Interact 195:206–214. doi:10.1016/j.cbi.2011.12.004
Hill RA, Connolly JD (2012) Triterpenoids. Nat Prod Rep 29:780. doi:10.1039/c2np20027a
Ip BC, Hu KQ, Liu C et al (2013) Lycopene metabolite, apo-10′-lycopenoic acid, inhibits diethylnitrosamine-initiated, high fat diet-promoted hepatic inflammation and tumorigenesis in mice. Cancer Prev Res 6:1304–1316. doi:10.1158/1940-6207.CAPR-13-0178
Jagan S, Ramakrishnan G, Anandakumar P et al (2008) Antiproliferative potential of gallic acid against diethylnitrosamine-induced rat hepatocellular carcinoma. Mol Cell Biochem 319:51–59. doi:10.1007/s11010-008-9876-4
James JT, Dubery IA (2009) Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) Urban. Molecules 14:3922–3941
Jang M, Cai L, Udeani GO et al (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 80(275):218–220. doi:10.1126/science.275.5297.218
Jayakumar S, Madankumar A, Asokkumar S et al (2012) Potential preventive effect of carvacrol against diethylnitrosamine-induced hepatocellular carcinoma in rats. Mol Cell Biochem 360:51–60. doi:10.1007/s11010-011-1043-7
Khan R, Kazmi I, Afzal M et al (2015) Fixed dose combination therapy loperamide and niacin ameliorates diethylnitrosamine-induced liver carcinogenesis in albino Wistar rats. RSC Adv 5:67996–68002. doi:10.1039/c5ra11201j
Kumar V, Verma A, Ahmed D et al (2013) Fostered antiarthritic upshot of Moringa oleifera lam. stem bark extract in diversely induced arthritis in wistar rats with plausible mechanism. Int J Pharm Sci Res 4:3894–3901. doi:10.13040/IJPSR.0975-8232.4(10).3894-01
Kumar V, Anwar F, Verma A, Mujeeb M (2014) Therapeutic effect of umbelliferon-α-d-glucopyranosyl-(2I→1II)-α-d-glucopyranoside on adjuvant-induced arthritic rats. J Food Sci Technol 52:3402–3411. doi:10.1007/s13197-014-1403-x
Kumar V, Al-Abbasi FA, Ahmed D et al (2015) Paederia foetida Linn. inhibits adjuvant induced arthritis by suppression of PGE 2 and COX-2 expression via nuclear factor-κB. Food Funct 6:1652–1666. doi:10.1039/c5fo00178a
Kumar V, Bhatt PC, Rahman M et al (2016) Melastoma malabathricum Linn attenuates complete freund’s adjuvant-induced chronic inflammation in Wistar rats via inflammation response. BMC Complement Altern Med 16:510. doi:10.1186/s12906-016-1470-9
Kwak MK, Itoh K, Yamamoto M et al (2001) Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3H-1, 2-dimethiole-3-thione. Mol Med 7:135–145
Kweon S, Park KA, Choi H (2003) Chemopreventive effect of garlic powder diet in diethylnitrosamine-induced rat hepatocarcinogenesis. Life Sci 73:2515–2526. doi:10.1016/S0024-3205(03)00660-X
Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect, Biol, p 1
Lawrence TS, Robertson JM, Anscher MS et al (1995) Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys 31:1237–1248. doi:10.1016/0360-3016(94)00418-K
Li J-J, Tang Q, Li Y et al (2006) Role of oxidative stress in the apoptosis of hepatocellular carcinoma induced by combination of arsenic trioxide and ascorbic acid. Acta Pharmacol Sin 27:1078–1084. doi:10.1111/j.1745-7254.2006.00345.x
Liu X, Dewaele S, Vanhooren V et al (2010) Alteration of N-glycome in diethylnitrosamine-induced hepatocellular carcinoma mice: a non-invasive monitoring tool for liver cancer. Liver Int 30:1221–1228. doi:10.1111/j.1478-3231.2010.02279.x
Mahato SB, Nandy AK, Roy G (1992) Triterpenoids. Phytochemistry 31:2199–2249
Majumder S, Roy S, Kaffenberger T et al (2010) Loss of metallothionein predisposes mice to diethylnitrosamine-induced hepatocarcinogenesis by activating NF-κB target genes. Cancer Res 70:10265–10276. doi:10.1158/0008-5472.CAN-10-2839
Marnewick JL, van der Westhuizen FH, Joubert E et al (2009) Chemoprotective properties of rooibos (Aspalathus linearis), honeybush (Cyclopia intermedia) herbal and green and black (Camellia sinensis) teas against cancer promotion induced by fumonisin B1 in rat liver. Food Chem Toxicol 47:220–229. doi:10.1016/j.fct.2008.11.004
Mbimba T, Awale P, Bhatia D et al (2012) Alteration of hepatic proinflammatory cytokines is involved in the resveratrol-mediated chemoprevention of chemically-induced hepatocarcinogenesis. Curr Pharm Biotechnol 13:229–234. doi:10.2174/138920112798868575
Naik SR, Thakare VN, Patil SR (2011) Protective effect of curcumin on experimentally induced inflammation, hepatotoxicity and cardiotoxicity in rats: evidence of its antioxidant property. Exp Toxicol Pathol 63:419–431. doi:10.1016/j.etp.2010.03.001
Neto CC (2007) Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases. Mol Nutr Food Res 51:652–664
Pradeep K, Mohan CVR, Gobianand K, Karthikeyan S (2007) Silymarin modulates the oxidant-antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur J Pharmacol 560:110–116. doi:10.1016/j.ejphar.2006.12.023
Pradeep K, Mohan CVR, Gobianand K, Karthikeyan S (2010) Protective effect of Cassia fistula Linn. on diethylnitrosamine induced hepatocellular damage and oxidative stress in ethanol pretreated rats. Biol Res 43:113–125. doi:10.4067/S0716-97602010000100013
Qi Y, Chen X, Chan CY et al (2008) Two-dimensional differential gel electrophoresis/analysis of diethylnitrosamine induced rat hepatocellular carcinoma. Int J Cancer 122:2682–2688. doi:10.1002/ijc.23464
Setzer WN, Setzer MC (2003) Plant-derived triterpenoids as potential antineoplastic agents. Mini Rev Med Chem 3:540–556. doi:10.2174/1389557033487854
Shaban NZ, El-Kersh MAL, El-Rashidy FH, Habashy NH (2013) Protective role of Punica granatum (pomegranate) peel and seed oil extracts on diethylnitrosamine and phenobarbital-induced hepatic injury in male rats. Food Chem 141:1587–1596. doi:10.1016/j.foodchem.2013.04.134
Shizuma T, Ishiwata K, Nagano M et al (2011) Protective effects of fermented rice vinegar sediment (Kurozu moromimatsu) in a diethylnitrosamine-induced hepatocellular carcinoma animal model. J Clin Biochem Nutr 49:31–35. doi:10.3164/jcbn.10-112
Sivalokanathan S, Ilayaraja M, Balasubramanian MP (2004) Anticancer potency of Terminalia arjuna bark on N-nitrosodiethylamine- induced hepatocellular carcinoma in rats. Nat Prod Sci 10:190–195. doi:10.1007/s11010-006-0433-8
Thangavel P, Vaiyapuri M (2013) Antiproliferative and apoptotic effects of naringin on diethylnitrosamine induced hepatocellular carcinoma in rats. Biomed Aging Pathol 3:59–64. doi:10.1016/j.biomag.2013.01.006
V.K, F.A. A-A, A.V, et al (2015) Umbelliferone β-d-galactopyranoside exerts an anti-inflammatory effect by attenuating COX-1 and COX-2. Toxicol Res (Camb) 4:1072–1084. doi:10.1039/c5tx90017d
Verma A, Bhatt PC, Kaithwas G et al (2016) Chemomodulatory effect Melastoma malabathricum Linn against chemically induced renal carcinogenesis rats via attenuation of inflammation, oxidative stress, and early markers of tumor expansion. Inflammopharmacology 24:233–251. doi:10.1007/s10787-016-0276-1
Verma A, Ahmed B, Anwar F et al (2017) Novel glycoside from Wedelia calendulacea inhibits diethyl nitrosamine-induced renal cancer via downregulating the COX-2 and PEG2 through nuclear factor-κB pathway. Inflammopharmacology 25:159–175. doi:10.1007/s10787-017-0310-y
Wagner JS, Adson MA, Van Heerden JA et al (2001) The natural history of hepatic metastases from colorectal cancer. A comparison with resective treatment. Ann Surg 199:502–508
Yin C, Evason K (2013) Hepatic stellate cells in liver development, regeneration, and cancer. J Cin Investig 123:1902–1910. doi:10.1172/JCI66369.1902
Yuan C, Wang C, Wang J et al (2016) Inhibition on the growth of human MDA-MB-231 breast cancer cells in vitro and tumor growth in a mouse xenograft model by Se-containing polysaccharides from Pyracantha fortuneana. Nutr Res 36:1243–1254. doi:10.1016/j.nutres.2016.09.012
Zhang CL, Zeng T, Zhao XL et al (2012) Protective effects of garlic oil on hepatocarcinoma induced by N-nitrosodiethylamine in rats. Int J Biol Sci 8:363–374. doi:10.7150/ijbs.3796
Zhao X, Chen Q, Li Y et al (2015) Doxorubicin and curcumin co-delivery by lipid nanoparticles for enhanced treatment of diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur J Pharm Biopharm 93:27–36. doi:10.1016/j.ejpb.2015.03.003
Acknowledgements
We gratefully acknowledge the Department of Pharmaceutical Sciences, Faculty of Health Sciences, Sam Higginbottom University of Agriculture, Technology and Sciences (SHUATS) for providing the facility for conducting the experimental study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verma, A., Singh, D., Anwar, F. et al. Triterpenoids principle of Wedelia calendulacea attenuated diethynitrosamine-induced hepatocellular carcinoma via down-regulating oxidative stress, inflammation and pathology via NF-kB pathway. Inflammopharmacol 26, 133–146 (2018). https://doi.org/10.1007/s10787-017-0350-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-017-0350-3