Abstract
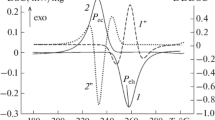
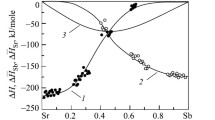
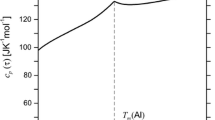
The enthalpy of Cs–Pb system alloys containing 40, 50, 60, and 66.67 at.% Pb have been measured and heat capacity have been determined using high-temperature drop calorimetry method over a temperature range 430–1075 K covering solid and liquid phases. For all alloys, recommended temperature dependences of the studied properties have been developed as well as the enthalpy changes on phase transitions and the liquidus temperature have been determined. The concentration dependence of the heat capacity of Cs–Pb liquid alloys at different temperatures has been constructed, at which the pronounced maximum is observed at a content of 50 at.% Pb. A significant excess of the heat capacity of the studied melts over additive values is revealed. It is shown that the results obtained are consistent with the assumptions in the literature about the formation of structural units with a partially ionic character of interatomic interaction in melts of the Cs–Pb system.







Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article.
References
V.F. Gantmakher, Usp. Fiz. Nauk (2002). https://doi.org/10.1070/PU2002v045n11ABEH001246
J.A. Meijer, G.J.B. Vinke, W. van der Lugt, J. Phys. F: Met. Phys. (1986). https://doi.org/10.1088/0305-4608/16/7/012
R.A. Khairulin, R.N. Abdullaev, S.V. Stankus, Phys. Chem. Liq. (2021). https://doi.org/10.1080/00319104.2020.1793335
H.T.J. Reijers et al., Phys. Rev. B (1989). https://doi.org/10.1103/PhysRevB.40.6018
H.T.J. Reijers, W. van Der Lugt, M.L. Saboungi, Phys. Rev. B (1990). https://doi.org/10.1103/PhysRevB.42.3395
M.L. Saboungi et al., J. Chem. Phys. (1988). https://doi.org/10.1063/1.455538
S.V. Stankus, I.V. Savchenko, O.S. Yatsuk, Instrum. Exp. Tech. (2017). https://doi.org/10.1134/S0020441217030265
S.V. Stankus, I.V. Savchenko, O.S. Yatsuk, High Temp. (2018). https://doi.org/10.1134/S0018151X18010170
S.V. Stankus, I.V. Savchenko, O.S. Yatsuk, E.P. Raschektaeva, Russ. J. Phys. Chem. A (2018). https://doi.org/10.1134/S0036024418090285
D.A. Ditmars, T.B. Douglas, J. Res. Natl. Bur. Stand., Sect. A (1971) https://doi.org/10.6028/jres.075A.031
L.V. Gurvich et al, Thermodynamic Properties of Pure Substances. Handbook: Vol. 4. Book 2 (Nauka, Moscow, USSR, 1982) [in Russian]
P.H. Sommelet, Gibbs Energies, Entropies and Heats of Formation from Drop Calorimetry: the Silver Lead System: M.S. Thesis (Berkley University, 1965)
R.N. Abdullaev, R.A. Khairulin, Y.M. Kozlovskii et al., Int. J. Thermophys. (2023). https://doi.org/10.1007/s10765-023-03187-1
M.H. Buschmann, Int. J. Thermophys. (2024). https://doi.org/10.1007/s10765-023-03295-y
Funding
This work was supported by the state contract with IT SB RAS (121031800219-2).
Author information
Authors and Affiliations
Contributions
ARK: writing and measurements; SVS: writing and supervision; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khairulin, A.R., Stankus, S.V. Enthalpy and Heat Capacity of Cs–Pb Alloys in Solid and Liquid States. Int J Thermophys 45, 67 (2024). https://doi.org/10.1007/s10765-024-03362-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-024-03362-y