Abstract
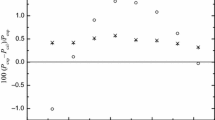
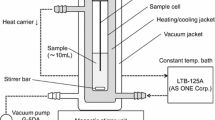
The present work falls within the research line focused on the phase equilibria of binary mixtures comprising typical compounds, widely present in biodiesel/petrodiesel blends. The study was performed by investigating the liquid–vapor equilibria and thermodynamic properties of two binary systems: ethyl octanoate (1) + n-tetradecane (2) and ethyl dodecanoate (1) + n-tetradecane (2) using experimental and computational methods. The experimental data of vapor pressures were measured through a static apparatus at nine temperatures from (373.15 K and 453.15 K) with an interval of 10 K. The obtained experimental data are used to determine the activity coefficients (γi) and excess molar Gibbs energies (GE) of the investigated binary mixtures by applying the Redlich–Kister equation according to Barker’s method. Positive values of the excess molar Gibbs energy are obtained for all the investigated constant temperatures and over the whole composition range. The obtained GE values were correlated by using two semi-predictive models (NRTL and Heil). The prediction displays agreement with the experimental data obtained with Barker’s method. The present work provides a set of thermodynamic data that may be very useful for the development of eco-friendly alternative biodiesel.








Similar content being viewed by others
Data Availability
All data supporting the findings of this study are available within the article.
References
M. Aghbashlo, W. Peng, M. Tabatabaei, S.A. Kalogirou, S. Soltanian, H.H.Z. Bandbafha, O. Mahian, S.S. Lam, Prog. Energy Combust. Sci. 85, 100904 (2021)
E.E.S. Michaelides, Alternative energy sources (Springer, New York, 2012)
W.V. Reid, M.K. Ali, C.B. Field, Glob. Chan. Biol. 26, 274 (2020)
A. Demirbas, Energy Policy 35, 4661 (2007)
M.H. Hassan, M.A. Kalam, Procedia Eng. 56, 39 (2013)
G.M. Mathew, D. Raina, V. Narisetty, V. Kumar, S. Saran, A. Pugazhendi, R. Sindhu, A. Pandey, P. Binod, Sci. Total. Environ. 794, 148751 (2021)
I.P. De Oliveira, A.R.L. Caires, Renew. Energy 140, 203 (2019)
M. Canakci, Bioresour. Technol. 98, 1167 (2007)
S. Lahane, K.A. Subramanian, Fuel 139, 537 (2015)
Y. Palani, C. Devarajan, D. Manickam, S. Thanikodi, Environ. Eng. Res. 27 (2022)
A.O. Guimaraes, F. Machado, E.C. Da Silva, A.M. Mansanares, Int. J. Thermophys. 33, 1842 (2012)
M. Benziane, K. Khimeche, I. Mokbel, A. Dahmani, J. Jose, J. Chem. Eng. Data 58, 492 (2013)
S. Chabane, M. Benziane, K. Khimeche, S. Didaoui, I. Mokbel, D. Trache, J. Jose, N. Yagoubi, J. Chem. Thermodyn. 113, 107 (2017)
M. Benziane, K. Khimeche, I. Mokbel, D. Trache, N. Yagoubi, J. Jose, J. Therm. Anal. Calorim. 126, 845 (2016)
M. Benziane, K. Khimeche, I. Mokbel, T. Sawaya, A. Dahmani, J. Jose, J. Chem. Eng. Data 56, 4736 (2011)
Z. Bouzina, A. Negadi, F. Dergal, I. Mokbel, J. Jose, L. Negadi, J. Mol. Liq. 201, 83 (2015)
N.C.B. Ahmed, L. Negadi, I. Mokbel, A.A. Kaci, J. Jose, J. Chem. Thermodyn. 44, 116 (2012)
L. Sahraoui, K. Khimeche, I. Mokbel, M. Benziane, J. Jose, J. Chem. Eng. Data 62, 1842 (2017)
I. Mokbel, A. Razzouk, T. Sawaya, J. Jose, J. Chem. Eng. Data 54, 819 (2009)
H. Li, K. Luo, S. Xia, P. Ma, Fluid Phase Equilib. 408, 47 (2016)
O. Redlich, A.T. Kister, J. Ind. Eng. Chem. 40, 345 (1948)
J.A. Barker, Aust. J. Chem. 6, 207 (1953)
J.C. Davidtz, Miner. Eng. 12, 1147 (1999)
S. Gebreyohannes, B.J. Neely, K. Gasem, Ind. Eng. Chem. Res. 53, 12445 (2014)
A.G. Carr, J.W. Tester, Fluid Ph. Equilib. 337, 288 (2013)
H. Renon, J.M. Prausnitz, AIChE J. 14, 135 (1968)
G.M. Wilson, J. Am. Chem. Soc. 86, 127 (1964)
J.F. Heil, J.M. Prausnitz, AIChE J. 12, 678 (1966)
I. Nagata, T. Ohta, Y. Uchiyama, J. Chem. Eng. Data 18, 54 (1973)
A.A. Touazi, S. Didaoui, K. Khimeche, M. Benziane, Thermochim. Acta 685, 178536 (2020)
A.A. Touazi, S. Didaoui, K. Khimeche, M. Rial, S.A. Moulai, M. Benziane, J. Mol. Liq. 327, 114860 (2021)
G.G. Benge, A comparison of thermodynamic models for the prediction of phase behavior in aqueous-polymer two-phase systems, Doctoral dissertation, Virginia Polytechnic Institute and State University, Blacksburg (1986)
S.V. Freitas, Â. Santos, M.L.C. Moita, L.A. Follegatti-Romero, T.P. Dias, A.J. Meirelles, J.A. Coutinho, Fuel 108, 840 (2013)
L. Zhang, Y. Guo, H. Wei, F. Yang, W. Fang, R. Lin, J. Chem. Eng. Data 55, 4108 (2010)
Funding
The authors declare that they have received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
FC: Conceptualization, Methodology, Resources, Investigation, data treatment, Writing-Original Draft. MB: Conceptualization, Methodology, Resources, Investigation, Writing-Original Draft. DT: Conceptualization, Review & Editing. AAT, IM, and JJ: Review & Editing the manuscript draft. All authors have approved the initial version of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval
All authors state that they adhere to the Ethical Responsibilities of Authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chalghoum, F., Benziane, M., Trache, D. et al. Vapor Pressure Measurements and Predictions for Binary Systems Containing Ethyl Octanoate or Ethyl Dodecanoate as Biodiesels and n-Tetradecane as Petrodiesel Compound. Int J Thermophys 44, 157 (2023). https://doi.org/10.1007/s10765-023-03265-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-023-03265-4