Abstract
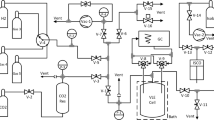
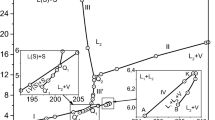
At the former IMGC-CNR (now INRiM), the Italian national metrological Institute, a series of pure hydrocarbons were measured in past years to determine with high accuracy for the temperature of their triple point and of their solid-to-solid transition(s) when present. While results on methane, ethane, and propane were published; the 1982 measurements on n-butane were never so far. It seems useful to add these results now to the still scarce existing data for possible inclusion in reference data archives. The paper summarises the experimental setup and results, compared with previous determinations. For the triple point, a temperature Ttp,90 = 134.869(2) K was found showing a higher accuracy. For the s.s.t.-I, the temperature was T90,upper-end = 108.2(3) K, where the low accuracy is due to the peculiar s.s.t. properties.


Similar content being viewed by others
Data Availability
Dataset: F. Pavese (1982). Author Archives. Available, on request to the Author, only in graphical form: chart registrations with handwritten info and comments.
References
F. Pavese, Metrologia 15, 47 (1979)
F. Pavese, J. Chem. Thermodyn. 10, 369 (1978)
F. Pavese, L.M. Besley, J. Chem. Thermodyn. 13, 1095 (1981)
F. Pavese, J. Chem. Thermodyn. 165, 106639 (2022)
F. Pavese, G. Cagna, D. Ferri, Proc. 6th Int. Cryo. Engin. Conf. (ICEC6) (IPC Science and Tech. Press, 1976), Guildford, 205.
F. Pavese, Temperature, Its Measurement and Control in Science and Industry, J.F. Schooley (Amer. Inst. of Phys., New York, 1983) 5, 209.
F. Pavese, G.F. Molinar, Modern Gas-Based Temperature and Pressure Measurements, 2nd edn., International Monograph Series on Cryogenic Engineering (Plenum Publ. Co., New York, 2013) pp. 650.
F. Pavese, Temperature, Its Measurement and Control in Science and Industry, D. Ripple (Amer. Inst. of Phys., New York, 2003) 8, 167.
J.G. Aston, G.H. Messerly, J. Am. Chem. Soc. 62, 1917 (1940)
H.M. Huffman, G.S. Parks, M. Barmore, J. Am. Chem. Soc. 53, 3876 (1931)
G. Klipping, F. Schmidt, Kältetechnik 17, 382–384 (1965)
W.M. Haynes, R.D. Goodwin, Thermophysical properties of normal butane from 135 to 700K at pressures to 70 MPa, NBS Monogr. (U. S.), 1982, 197 pp.
B.A. Younglove, J.F. Ely, Thermophysical Properties of Fluids II. Methane, Ethane, Propane, Isobutane, and Normal Butane, J. Phys. Chem. Ref. Data, 1987, 16, 577.
E.K. Lang, Thesis, The University of British Columbia (Vancouver), 2013.
NIST Chemistry WebBook SRD 69, last update 2023. https://webbook.nist.gov/chemistry/, https://doi.org/10.18434/T4D303 .
H. Miyamoto, K. Watanabe, Int. J. Thermophys. 22, 459 (2001)
K. Refson, G.S. Pawley, Acta Cryst. B42, 402 (1986)
Funding
No funding received.
Author information
Authors and Affiliations
Contributions
The author is the single contributor.
Corresponding author
Ethics declarations
Competing Interests
The author has no conflicts to disclose.
Ethical Approval
No animal or human participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pavese, F. The Triple Point and Solid-to-Solid Phase Transition at ≈ 107 K of n-Butane. Int J Thermophys 44, 119 (2023). https://doi.org/10.1007/s10765-023-03228-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-023-03228-9