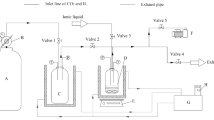
Abstract
The working pairs composed of hydrofluoroolefins (HFOs) and ionic liquids (ILs) have exhibited promising potential in the absorption refrigeration systems (ARS). In order to exploit the possibility of 3,3,3-trifluoropropene/ILs as the pairs used in the ARS, the experimental determination on the solubility of 3,3,3-trifluoropropene in 1-hexyl-3-methyl-imidazolium trifluoromethanesulfonate ([hmim][TfO]) and 1-octyl-3-methyl-imidazolium trifluoromethanesulfonate ([omim][TfO]) ILs was conducted. The present measurements were carried out based on the isochoric saturation method at the temperature range from 303.15 K to 343.15 K. The experimental solubility data were correlated through the non-random two-liquid (NRTL) model, universal quasi-chemical (UNIQUAC) model and Krichevsky-Kasarnovsky (K-K) model, respectively. In addition, the influence of the alkyl chain length for different ILs on the solubility of R1243zf and the dissolving capacities of different HFOs in [hmim][TfO] were compared. The Henry’s constants and mixing thermodynamic properties (enthalpy, entropy and Gibbs Energy) of R1243zf in [hmim][TfO] and [omim][TfO] were calculated and discussed. Furthermore, the coefficients of performance of different R1243zf/IL working pairs in single-effect ARS were compared.













Similar content being viewed by others
Data Availability
Not applicable.
References
G. Kaur, H. Kumar, M. Singla, J. Mol. Liq. 351, 118556 (2022)
A. Berthod, M.J. Ruiz-Angel, S. Carda-Broch, J. Chromatogr. A 1559, 2–16 (2018)
H. Olivier-Bourbigou, M. Magna, D. Morvan, Appl. Catal. A-Gen. 373, 1–56 (2010)
D. Wei, A. Ivaska, Anal. Chim. Acta 607, 126–135 (2008)
M. Khamooshi, K. Parham, U. Atikol, Adv. Mech. Eng. 5, 620592 (2013)
Y.R. Sui, W. Wu, Energy 263, 125689 (2023)
G. Takalkar, A.K. Sleiti, Front. Energy 16, 521–535 (2022)
A. Mehari, Z.Y. Xu, R.Z. Wang, Energ. Convers Manage 206, 112482 (2020)
X.Y. Liu, L.H. Bai, S.Q. Liu, M.G. He, J. Chem. Eng. Data 61, 3952–3957 (2016)
Y. Zhang, J.J. Yin, X.P. Wang, J. Mol. Liq. 260, 203–208 (2018)
S. Asensio-Delgado, F. Pardo, G. Zarca, A. Urtiaga, J. Chem. Eng. Data 65, 4242–4251 (2020)
S. Asensio-Delgado, F. Pardo, G. Zarca, A. Urtiaga, Sep. Purif. Technol. 249, 117136 (2020)
S. Asensio-Delgado, M. Viar, F. Pardo, G. Zarca, A. Urtiaga, Fluid Phase Equilib. 549, 113210 (2021)
S. Asensio-Delgado, M. Viar, A.A.H. Padua, G. Zarca, A. Urtiaga, A.C.S. Sustain, Chem. Eng. 10, 15124–15134 (2022)
X.P. Wang, Y. Zhang, D.B. Wang, Y.J. Sun, J. Chem. Eng. Data 62, 1825–1831 (2017)
Y.J. Sun, Y. Zhang, G.L. Di, X.P. Wang, J.M. Prausnitz, L.W. Jin, J. Chem. Eng. Data 63, 3053–3060 (2018)
T. Jiang, X.Z. Meng, Y.J. Sun, L.J. Jin, Q.M. Wei, J. Wang, X.P. Wang, M.G. He, Int. J. Refrig. 131, 178–185 (2021)
Y.J. Sun, Y. Zhang, X.P. Wang, J.M. Prausnitz, L.W. Jin, Fluid Phase Equilib. 450, 65–74 (2017)
Y. Zhang, X.C. Jia, X.P. Wang, Int. J. Refrig. 117, 338–345 (2020)
X.Y. Liu, P. Pan, S.G. Peng, M.G. He, Y.D. He, CIESC J. 68, 4486–4493 (2018)
M.G. He, P. Pan, F. Yang, T. Wang, X.Y. Liu, J. Chem. Eng. Data 63, 1780–1788 (2018)
W. Wu, H. Zhang, T. You, X. Li, Ind. Eng. Chem. Res. 56, 9906–9916 (2017)
Y.J. Sun, G.L. Di, J. Wang, X.P. Wang, W. Wu, Int. J. Refrig. 109, 25–36 (2020)
J.M. Asensio-Delgado, S. Asensio-Delgado, G. Zarca, A. Urtiaga, Int. J. Refrig. 134, 232–241 (2022)
X.Y. Liu, Z. Ye, L.H. Bai, M.G. He, Energ. Convers Manage 181, 319–330 (2019)
N.C. Zhang, Y.D. Dai, Int. J. Thermophys. 42, 152 (2021)
N.A. Lai, Appl. Therm. Eng. 70, 1–6 (2014)
V. Nair, Int. J. Refrig. 122, 156–170 (2021)
R. Ciconkov, Int. J. Refrig. 86, 441–448 (2018)
X.C. Jia, W.B. Dou, X.P. Wang, J. Mol. Liq. 364, 120031 (2022)
X.C. Jia, H. Wang, X.P. Wang, J. Chem. Thermodyn. 164, 106637 (2022)
X.C. Jia, Y. Luo, X.P. Wang, J. Mol. Liq. 347, 118347 (2022)
X.C. Jia, Y.T. Ma, X.P. Wang, J. Mol. Liq. 372, 121228 (2023)
A.M. Sadanandhan, P.K. Khatri, S.L. Jain, J. Mol. Liq. 295, 111722 (2019)
N.A. Noorhisham, D. Amri, A.H. Mohamed, N. Yahaya, N.M. Ahmad, S. Mohamad, S. Kamaruzaman, H. Osman, J. Mol. Liq. 326, 115340 (2021)
J.E. Sosa, R.P.P.L. Ribeiro, P.J. Castro, J.P.B. Mota, J.M.M. Araujo, A.B. Pereiro, Ind. Eng. Chem. Res. 58, 20769–20778 (2019)
M.L. Ferreira, N.S.M. Vieira, P.J.N. Castro, L.F. Vega, A.B. Pereiro, J.M.M. Araujo, J. Mol. Liq. 359, 119285 (2022)
M.C. Bubalo, K. Radosevic, I.R. Redovnikovic, J. Halambek, V.G. Srcek, Ecotox. Environ. Safe. 99, 1–12 (2014)
J. Flieger, M. Flieger, Int. J. Mol. Sci. 21, 6267 (2020)
S. Gehrke, M. von Domaros, R. Clark, O. Holloczki, M. Brehm, T. Welton, A. Luzar, B. Kirchner, Faraday Discuss. 206, 219–245 (2018)
A.B. Pereiro, J.M.M. Araujo, S. Martinho, F. Alves, S. Nunes, A. Matias, C.M.M. Duarte, L.P.N. Rebelo, I.M. Marrucho, A.C.S. Sustain, Chem. Eng. 1, 427–439 (2013)
B.L. Shi, J. Mol. Liq. 320, 114412 (2020)
S. Stolte, S. Abdulkarim, J. Arning, A.K. Blomeyer-Nienstedt, U. Bottin-Weber, M. Matzke, J. Ranke, B. Jastorff, J. Thöming, Green Chem. 10, 214–224 (2008)
E.W. Lemmon, I.H. Bell, M.L. Huber, M.O. McLinden, NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 10.0, National Institute of Standards and Technology, 2019.
H. Renon, J.M. Prausnitz, AIChE J. 14, 135–144 (1968)
A. Kamgar, F. Esmaeilzadeh, J. Mol. Liq. 220, 631–634 (2016)
I.R. Krichevsky, J.S. Kasarnovsky, J. Am. Chem. Soc. 57, 2168–2171 (1935)
J.M. Smith, H.C.V. Ness, M.M. Abbott, Introduction to Chemical Engineering Thermodynamics, 6th edn. (McGraw-Hill, New York, 2002)
K. Dong, Q. Wang, X.M. Lu, Q. Zhou, S.J. Zhang, Struct. Bond. 151, 1–38 (2014)
Funding
This work was supported by the National Key Research and Development Program of China (Grant No. 2022YFE0210200) and National Natural Science Foundation of China (No. 51936009).
Author information
Authors and Affiliations
Contributions
XJ contributed to measuring the solubilities, writing draft version. LM contributed to the correlation of the models and analysis. XW contributed to reviewing and editing the whole manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, X., Ma, L. & Wang, X. Experimental Investigation on the Solubility of 3,3,3-Trifluoropropene in [hmim][TfO] and [omim][TfO] ILs from 303.15 K to 343.15 K. Int J Thermophys 44, 92 (2023). https://doi.org/10.1007/s10765-023-03200-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-023-03200-7