Abstract
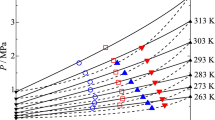
Mixing R13I1 with flammable or weakly flammable refrigerants is an approach to reduce both their flammability and global warming potential (GWP); however, thermodynamic property data of mixtures containing R13I1 are currently scarce. In this work, experimental measurements were performed for the \((p, \rho , T)\) behavior in the vapor-phase of R32 + R13I1, R125 + R13I1, and R32 + R125 + R13I1 mixtures. The measurements were made at temperatures from 300 K to 400 K and pressures up to 6.3 MPa, and 44 data points were obtained for the R32 + R13I1 mixture and 25 data points for the R125 + R13I1 mixture. For the ternary mixture, 25 data points were obtained at the composition of R466A (R32:R125:R13I1 = 49:11.5:39.5 \(\mathrm {mass\%}\)). The validity of the experimental data was verified by comparing to the virial equation of state generalized for nonpolar or weakly polar fluids based on the corresponding states principle. The comparison showed that deviations between the experimental and calculated pressures are generally minor and less scattered; this suggests that the \((p, \rho , T)\) data obtained in this work are reasonable in terms of the law of corresponding states.








Similar content being viewed by others
Data Availability
Not applicable.
References
ANSI/ASHRAE Standard 34-2019; Designation and Safety Classification of Refrigerants (2019)
K. Schultz, in 25th IIR International Congress of Refrigeration, August 24–30, Montreal. Canada 2019, 515–522 (2019). https://doi.org/10.18462/iir.icr.2019.0134
S. Kujak, K. Schultz, in 25th IIR International Congress of Refrigeration, August 24–30, Montreal. Canada 2019, 633–640 (2019). https://doi.org/10.18462/iir.icr.2019.0227
Z. Yang, B. Feng, H. Ma, L. Zhang, C. Duan, B. Liu, Y. Zhang, S. Chen, Z. Yang, Int. J. Refrig. 126, 12 (2021). https://doi.org/10.1016/j.ijrefrig.2021.01.022
A.G. Devecioğlu, V. Oruç, Eng. Sci. Technol. Int. J. 23, 1425 (2020). https://doi.org/10.1016/j.jestch.2020.04.003
E.W. Lemmon, I.H. Bell, M.L. Huber, M.O. McLinden. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 10.0, National Institute of Standards and Technology (2018). https://doi.org/10.18434/T4JS3Chttps://www.nist.gov/srd/refprop
E.W. Lemmon, R. Span, J. Chem. Eng. Data 60, 3745 (2015). https://doi.org/10.1021/acs.jced.5b00684
N. Sakoda, Y. Higashi, in Proceedings of the 20th Symposium on Thermophysical Properties (Boulder, CO, USA) (2021)
Y. Kayukawa, C. Kondou, N. Sakoda, K. Kariya, S. Fukuda, JSRAE Thermodynamic Tables JARef Vol. 5: HFOs & HCFOs (Japan Society of Refrigerating and Air Conditioning Engineers, 2021)
Y. Higashi, S. Hayasaka, C. Shirai, R. Akasaka, Int. J. Refrig. 52, 100 (2015). https://doi.org/10.1016/j.ijrefrig.2014.12.007
Y. Higashi, N. Sakoda, J. Chem. Eng. Data 63, 3818 (2018). https://doi.org/10.1021/acs.jced.8b00452
R. Akasaka, Y. Higashi, N. Sakoda, S. Fukuda, E.W. Lemmon, Int. J. Refrig. 119, 457 (2020). https://doi.org/10.1016/j.ijrefrig.2020.07.011
R. Akasaka, S. Fukuda, K. Miyane, Y. Higashi, J. Chem. Eng. Data 67, 346 (2022). https://doi.org/10.1021/acs.jced.1c00890
Y. Higashi, S. Okazaki, Y. Takaishi, M. Uematsu, K. Watanabe, J. Chem. Eng. Data 29, 31 (1984). https://doi.org/10.1021/je00035a012
C. Tsonopoulos, AIChE J. 20, 263 (1974). https://doi.org/10.1002/aic.690200209
L. Weber, Int. J. Thermophys. 15, 461 (1994). https://doi.org/10.1007/BF01563708
R. Tillner-Roth, A. Yokozeki, J. Phys. Chem. Ref. Data 26, 1273 (1997). https://doi.org/10.1063/1.556002
H. Orbey, J. Vera, AIChE J. 29, 107 (1983). https://doi.org/10.1002/aic.690290115
E.W. Lemmon, R. Jacobsen, J. Phys. Chem. Ref. Data 34, 69 (2005). https://doi.org/10.1063/1.1797813
Y.Y. Duan, M.S. Zhu, L. Shi, L.Z. Han, Fluid Phase Equilib. 131, 233 (1997). https://doi.org/10.1016/S0378-3812(96)03227-X
K. Tanaka, Netsu Bussei 35, 140 (2021). (in Japanese)
P. Chueh, J. Prausnitz, AIChE J. 13, 896 (1967). https://doi.org/10.1002/aic.690130516
E.W. Lemmon, R.T. Jacobsen, J. Phys. Chem. Ref. Data 33, 593 (2004). https://doi.org/10.1063/1.1649997
Acknowledgements
This work was based on results obtained in a project commissioned by New Energy and Industrial Technology Development Organization (NEDO). The authors appreciate Eric W. Lemmon, National Institute of Standards and Technology, Boulder, for his assistance during the documentation of this paper.
Funding
This work is based on results obtained from a project, P18005, commissioned by the New Energy and Industrial Technology Development Organization (NEDO).
Author information
Authors and Affiliations
Contributions
YH contributed to measuring \((p, \rho , T)\) behavior, including evaluating their experimental uncertainties. YK contributed to the statistical analysis of deviations between experimental data and calculated values from the virial equation of state. RA contributed to writing, reviewing, and editing the whole manuscript, as well as depicting figures and tables.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akasaka, R., Kayukawa, Y. & Higashi, Y. Vapor-Phase \(\varvec{(p, \rho , T)}\) Behavior of Difluoromethane (R32) + Trifluoroiodomethane (R13I1), Pentafluoroethane (R125) + R13I1, and R32 + R125 + R13I1 Mixtures: Experimental Measurements Based on the Isochoric Method and Verification with a Generalized Virial Equation of State. Int J Thermophys 43, 159 (2022). https://doi.org/10.1007/s10765-022-03080-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-022-03080-3