Abstract
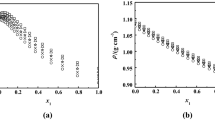
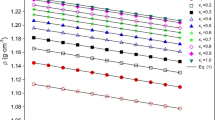
1,2-Ethanediol, 1,2-propanediol and their aqueous solutions are widely used as heat transfer fluids. Their thermal conductivity is a vital physical property, yet there are only few reports in literature. In this paper, thermal conductivity of binary aqueous solutions of the two glycols was measured using the transient hot wire method at temperature from 253.15 K to 373.15 K at atmospheric pressure. Measurement was made for six compositions over the entire concentration range from 0 mol to 1 mol fraction of glycol, namely, 0.0 mol, 0.2 mol, 0.4 mol, 0.6 mol, 0.8 mol, and 1.0 mol fraction of glycol. The uncertainties of temperature and concentration measurement were estimated to be 0.01 K and 0.1 %, respectively. The combined expanded uncertainty of thermal conductivity with a level of confidence of 0.95 (k = 2) was 2 %. The second-order Scheffé polynomial was used to correlate the temperature and composition dependence of the experimental thermal conductivity, which was found to be in good agreement with the experiment data from the present work and other reports.







Similar content being viewed by others
References
S. Rebsdat, D. Mayer, Ullmann’s Encyclopedia of Industrial Chemistry (WWiley, Hoboken, 2000). https://doi.org/10.1002/14356007.a10_101
C.J. Sullivan, Ullmann’s Encyclopedia of Industrial Chemistry (Wiley, Hoboken, 2000). https://doi.org/10.1002/14356007.a22_163.pub2
ASHRAE, Physical properties of secondary coolants (brines), in ASHRAE Handbook—Fundamentals (ASHRAE, Atlanta, 2005), pp. 31.1–31.13
F.N. Shamsetdinov, Z.I. Zaripov, I.M. Abdulagatov, M.L. Huber, F.M. Gumerov, F.R. Gabitov, A.F. Kazakov, Int. J. Refrig 36, 1347–1368 (2013)
M.S. Najjar, K.J. Bell, R.N. Maddox, Heat Transf. Eng. 2, 27–39 (1981)
D. Bohne, S. Fischer, E. Obermeier, Ber. Bunsenges. Phys. Chem. 88, 739–742 (1984)
K. Kroenlein, V. Diky, C.D. Muzny, R.D. Chirico, J.W. Magee, M. Frenkel, ThermoLit: NIST Literature Report Builder for Thermalchemical Properties Measurements (US Secretary of Commerce, 2016) http://trc.nist.gov/thermolit/main/home.html#home. Accessed 06 November 2017
O. Bates, G. Hazzard, Ind. Eng. Chem. 37, 193–195 (1945)
L. Riedel, Chem. Ing. Tech. 23, 465–469 (1951)
Y.L. Rastorguev, Y.A. Ganiev, Russ. J. Phys. Chem. 40, 869–871 (1966)
Y.L. Rastorguev, Y.A. Ganiev, Zh. Fiz. Khim. 41, 1352 (1967)
W.N. Vanderkooi, D.L. Hildenbrand, D.R. Stull, J. Chem. Eng. Data 12, 377–379 (1967)
Y.A. Ganiev, Y.L. Rastorguev, Russ. J. Phys. Chem. 42, 68–71 (1968)
I.U. Usmanov, A.S. Salikhov, Russ. J. Phys. Chem. 51, 1488–1489 (1977)
I.S. Bogacheva, K.B. Zemdikhanov, G.K. Mukhamedzyanov, A.K. Sadykov, A.G. Usmanov, Russ. J. Phys. Chem. 54, 838–839 (1980)
A. Grigrev, Thermal Conductivity: Water and Water-Organic Systems, Sov. Un. Gov. Bur. of Stand., Grozny Oil Inst., Inf. Ser. (1985)
M.J. Assael, E. Charitidou, S. Avgoustiniatos, W.A. Wakeham, Int. J. Thermophys. 10, 1127–1140 (1989)
T. Sun, A.S. Teja, J. Chem. Eng. Data 48, 198–202 (2003)
T. Sun, A.S. Teja, J. Chem. Eng. Data 49, 1311–1317 (2004)
X. Li, J. Wu, Q. Dang, J. Chem. Eng. Data 55, 1241–1246 (2010)
G. Labudová, V. Vozárová, J. Therm. Anal. Calorim. 67, 257–265 (2002)
X. Jin, Experimental Research of Heat Conductiv-ity for Liquids Based on Transient Hot-Wire Method (Xi’an Jiaotong Unvieristy, 2004) [unpublished Master’s thesis]
W.W. Focke, Int. J. Thermophys. 29, 1342–1360 (2008)
Y.A. Ganiev, Zh. Fiz. Khim. 43, 239–240 (1969)
Y.L. Rastorguev, M.A. Gazdiev, Inzh. Fiz. Zh. 17, 72–79 (1969)
R. DiGuilio, A.S. Teja, J. Chem. Eng. Data 35, 117 (1990)
M.J. Pastoriza-Gallego, L. Lugo, D. Cabaleiro, J.L. Legido, M.M. Piñeiro, J. Chem. Thermodyn. 73, 23–30 (2014)
G.M. Mallan, M.S. Michaelian, F.J. Lockhart, J. Chem. Eng. Data 17, 412 (1972)
J.C. Zhou, Y.Y. Che, K.J. Wu, J. Shen, C.H. He, J. Chem. Eng. Data 58, 663–670 (2013)
M.L. Huber, R.A. Perkins, D.G. Friend, J.V. Sengers, M.J. Assael, I.N. Metaxa, K. Miyagawa, R. Hellmann, E. Vogel, J. Phys. Chem. Ref. Data 41, (2012)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, C., Zhang, K. Thermal Conductivity of 1,2-Ethanediol and 1,2-Propanediol Binary Aqueous Solutions at Temperature from 253 K to 373 K. Int J Thermophys 42, 81 (2021). https://doi.org/10.1007/s10765-021-02837-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-021-02837-6