Abstract
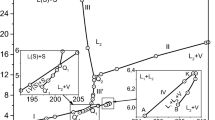
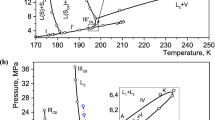
Precision data of the accurate calorimetric measurements of phase equilibria for ternary mixtures of methane, propane, and octane especially for the low concentration of octane have been presented. Based on the experimental data of heat capacity, internal energy, pressure, and temperature derivative of pressure at constant volume, the phase diagrams have been plotted in the range 140–350 K and 0.1–24 MPa. Phase transitions are localized by the finite discontinuities in temperature derivatives of the thermodynamic potentials. Our investigations show that hydrocarbon mixtures for the low concentration of octane split into two phases, the macrophase enriched by methane and propane and the octane-rich microphase. Besides, octane provokes a split of the liquid part of the macrophase into two liquid phases, the octane-rich phase and the octane-lean phase. To prove that all phases are equilibrium phases a cooling mode of measurements is used. At the cooling mode of measurements the same phase transitions as at the heating mode occur. These phase transitions correspond to the formation of the octane-rich microphase and the macrophase enriched by methane and propane. Besides, at the cooling mode of measurements a split of the liquid part of the macrophase into two liquid phases takes place.






Similar content being viewed by others
Notes
The word “bifurcation” means to divide or fork into two branches.
The word “isomorphism” derives from the Greek iso, meaning "equal," and morphosis, meaning "to form" or "to shape."
Abbreviations
- P :
-
Pressure [MPa]
- T :
-
Temperature [K]
- U :
-
Internal energy [J]
- Ω/V = -P :
-
Density of grand potential [MPa]
- C V = (δU/δT) V :
-
Heat capacity at constant volume [kJ·kg−1·K−1]
- (δP/δT) V :
-
Temperature derivative of pressure at constant volume (the thermal pressure coefficient) [MPa·K−1]
- (δP/δT) σ :
-
Temperature derivative of pressure along the equilibrium (saturation) curve [MPa·K−1]
- X :
-
Concentration [mole fraction]
- M :
-
Mass [kg]
- ρ :
-
Density [kg·m−3]
- V:
-
Vapor phase
- L:
-
Methane-propane liquid phase
- L1 :
-
Octane-lean liquid phase
- L2 :
-
Octane-rich liquid phase
- S:
-
Microphase
- UCEP:
-
Upper critical end point
- LCST:
-
Lower critical solution point
- BP:
-
Bifurcation point
- CPbinary :
-
Critical point of a binary mixture
- CP*binary :
-
Critical point of a binary mixture in the presence of an octane-rich microphase
References
V.M. Buleiko, D.V. Buleiko, Int. J. Thermophys. 41, 27 (2020). https://doi.org/10.1007/s10765-020-2602-5
V.M. Buleiko, B.A. Grigoriev, V.S. Muzykina, News Gas Sci. 1, 116 (2019). ((in Russian))
P.H. van Konynenburg, R.L. Scott, Philos. Trans. 298A, 445 (1980)
E.A. Guggenheim, Mixtures. The Theory of the Equilibrium Properties of Some Simple Classes of Mixtures, Solutions and Alloys (Oxford University Press, Amen Hous, London, 1952).
J.S. Rowlinson, F.L. Swinton, Liquids and Liquid Mixtures (Butterworth & Co (Publishers) Ltd, London, 1982).
A.J. Davenport, J.S. Rowlinson, Trans. Faraday Soc. 59, 78 (1963)
J.P. Kohn, Y.J. Kim, Y.C. Pan, J. Chem. Eng. Data 33, 278 (1966)
A.B. Rodrigues, J.P. Kohn, J. Chem, Eng. Data 12, 191 (1967)
J.R. Wagner, D.S. McCaffrey, J.P. Kohn, J. Chem. Eng. Data 13, 22 (1968)
K.S. Pedersen, A. Fredenslund, P. Thomassen, Properties of Oils and Gases. Contributions in Petroleum Geology and Engineering (Gulf Publishing Company, Houston, 1989).
V.M. Buleiko, B.A. Grigoriev, V.A. Istomin, Vestnik Kazan Technol. Univ. 17, 101 (2014). ((in Russian))
P.I. Freeman, J.S. Rowlinson, Pure Appl. Chem. (1961). https://doi.org/10.1351/pac196102010329
J.D. Hottovy, J.P. Kohn, K.D. Luks, J. Chem. Eng. Data 26, 135–137 (1981)
J.D. Hottovy, J.P. Kohn, K.D. Luks, J. Chem. Eng. Data 27, 298–302 (1982)
B. Lagourette, J.L. Daridon, J.F. Gaubert, H. Saint-Guirons, J. Chem. Thermodyn. 27, 259–266 (1995)
Z.M. Ramazanova, Izv. Vyssh. Uchebn. Zaved. Neft Gaz 7, 77–81 (1964)
V.M. Buleiko, B.A. Grigoriev, V.A. Istomin, Fluid Phase Equilib. 441, 64 (2017)
V.M. Buleiko, B.A. Grigoriev, J. Mendoza, Fluid Phase Equilib. 462, 14 (2018)
V.M. Buleiko, Laws governing the phase transformations of hydrocarbon mixtures in the oil and gas reservoirs of the developed deposits (based on the experimental data), Thesis of the scientific degree of Doctor of Technical Science. (Russian Academy of Science, 2007), (in Russian).
D. Frenkel, J. Phys.: Condens. Matter 6, 71 (1994)
I. Iosilevskiy, Non-Ideality and Phase Transitions in Coulomb Systems, Lambert (Academic Publishing, Berlin, 2011).
V. Gryaznov, I. Iosilevskiy, J. Phys. A: Math. Theor. 42, 214007 (2009)
I. Iosilevskiy, J. Phys. Conf. Ser. 653, 012077 (2015)
M. Hempel, O. Heinimann, A. Yudin, I. Iosilevskiy, M. Liebendoerfer, F.-K. Thielemann, Phys. Rev. D 94, 103001 (2016)
Acknowledgement
This work was supported by the Russian Foundation for Fundamental Research under Grant No. 19-08-00202.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buleiko, V.M., Buleiko, D.V. Phase Diagrams for Ternary Mixtures of Methane, Propane, and Octane for the Low Concentration of Octane. Int J Thermophys 42, 85 (2021). https://doi.org/10.1007/s10765-021-02835-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-021-02835-8