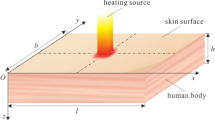
Abstract
Laser technology is increasingly used, and it is crucial for both safety and medical reasons that the impact of laser irradiation on human skin can be accurately predicted. This study is mainly focused on laser–skin interactions and potential lesions (burns). A mathematical model dedicated to heat transfers in skin exposed to infrared laser radiations has been developed. The model is validated by studying heat transfers in human skin and simultaneously performing experimentations an animal model (pig). For all experimental tests, pig’s skin surface temperature is recorded. Three laser wavelengths have been tested: 808 nm, 1940 nm and 10 600 nm. The first is a diode laser producing radiation absorbed deep within the skin. The second wavelength has a more superficial effect. For the third wavelength, skin is an opaque material. The validity of the developed models is verified by comparison with experimental results (in vivo tests) and the results of previous studies reported in the literature. The comparison shows that the models accurately predict the burn degree caused by laser radiation over a wide range of conditions. The results show that the important parameter for burn prediction is the extinction coefficient. For the 1940 nm wavelength especially, significant differences between modeling results and literature have been observed, mainly due to this coefficient’s value. This new model can be used as a predictive tool in order to estimate the amount of injury induced by several types (couple power-time) of laser aggressions on the arm, the face and on the palm of the hand.









Similar content being viewed by others
References
E.F. Maher, in Report SAM-TR-78-32: Transmission and absorption coefficients for ocular media of the Rhesus monkey (Brooks Air Force Base, San Antonio, TX, 1978)
N. Museux, L. Perez, L. Autrique, D. Agay, Burns 38, 658 (2012)
S.D. Jackson, A. Lauto, Lasers Surg. Med. 30, 184 (2002)
S.L. Jacques, Appl. Opt. 32, 2447 (1993)
B. Chen, D.C. O’Dell, S.L. Thomsen, B.A. Rockwell, A.J. Welch, Lasers Surg. Med. 37, 373 (2005)
B. Choi, J.K. Barton, E.K. Chan, A.J. Welch, Lasers Surg. Med. 23, 185 (1998)
B.M. Hantash, V.P. Bedi, B. Kapadia, Z. Rahman, K. Jiang, H. Tanner, K.F. Chan, Lasers Surg. Med. 39, 96 (2007)
B.P. Payne, N.S. Nishioka, B.B. Mikic, V. Venugopalan, Lasers Surg. Med. 23, 1 (1998)
A.J. Welch, J.H. Torres, W.F. Cheong, Physics, system design, experimental applications. Tex. Heart Inst. J. 16, 141 (1989)
T.P. Sullivan, W.H. Eaglstein, S.C. Davis, P. Mertz, Wound Rep. Reg. 9, 66 (2001)
H.H. Pennes, J. Appl. Physiol. 1, 93 (1948)
B. Chen, S.L. Thomsen, R.J. Thomas, J. Oliver, A.J. Welch, Lasers Surg. Med. 40, 358 (2008)
A.R. Moritz, F.C. Henriques, Am. J. Pathol. 23, 695 (1947)
F.F. Henriques, Arch. Pathol. 43, 489 (1947)
A. Shitzer, M.K. Kleiner, Thermal behavior of biological tissues—a general analysis. Bull. Math. Biol. 38, 369–386 (1976)
M.M. Chen, K.R. Holmes, Microvascular contributions in tissue heat transfer. Ann. N. Y. Acad. Sci. 335, 137–150 (1980)
S. Weinbaum, L.M. Jiji, D.E. Lemons, Theory and experiment for the effect of vascular microstructure on surface tissue heat transfer. Part I. ASME J. Biomech. Eng. 106, 321–330 (1984)
J. Lang, B. Erdmann, M. Seebass, Impact of nonlinear heat transfer on temperature control in regional hyperthermia. IEEE Trans. Biomed. Eng. 46, 1129–1138 (1999)
D.T. Tompkins, R. Vanderby, S.A. Klein, W.A. Beckman, R.A. Steeves, D.M. Frye, B.R. Paliwal, Int. J. Hyperth. 10, 517 (1994)
B. Erdmann, J. Lang, M. Seebass, Optimization of temperature distributions for regional hyperthermia based on a nonlinear heat transfer model. Ann. N. Y. Acad. Sci. 858, 36–46 (1998)
E. Kengne, I. Mellal, M.B. Hamouda, A. Lakhssassi, A mathematical model to solve bio-heat transfer problems through a bio-heat transfer equation with quadratic temperature-dependent blood perfusion under a constant spatial heating on skin surface. J. Biomed. Sci. Eng. 7, 721–730 (2014)
D. Fiala, K.J. Lomas, M. Stohrer, J. Appl. Physiol. 87, 1957 (1999)
J.P. Abraham, E.M. Sparrow, Int. J. Heat Mass Trans. 50, 2537 (2007)
M. Knudsen, J. Overgaard, I.E.E.E. Trans, Biomed. Eng. 33, 477 (1986)
C. Lormel, Ph.D. Thesis (Perpignan, France 2005)
H.S. Carslaw, J.C. Jaeger, Conduction of Heat in Solids, 2nd edn. (Oxford Science Publications, Oxford, 1946)
F.P. Incropera, D.P. Dewitt, Fundamentals of Heat and Mass Transfer, 5th edn. (Wiley, New York, 2001)
J.H. Torres, M. Motamedi, J.A. Pearce, A.J. Welch, Appl. Opt. 32, 597 (1993)
S.H. Diaz, G. Aguilar, E.J. Lavernia, B.J.F. Wong, IEEE J. Quantum Electron. 7, 944 (2001)
A. Takata, Aerosp. Med. 45, 634 (1974)
J.Z. Zhang, Y.G. Shen, X.X. Zhang, Lasers Med. Sci. 24, 329 (2009)
H.S. Hatfield, J. Physiol. 120, 35 (1953)
B. Chen, S.L. Thomsen, R.J. Thomas, A.J. Welch, J. Biomed. Opt. 11, 1 (2006)
L. Autrique, C. Lormel, Numerical design of experiment for sensitivity analysis – application to skin burn injury prediction. IEEE Trans. Biomed. Eng. 55(4), 1279–1290 (2008)
F. Breaban, J.F. Coutouly, P. Deprez, A. Deffontaine, Lasers Eng. 11, 77 (2001)
S. Mordon, in Groupe laser de la Société Française de Dermatologie : les lasers en dermatologie, 2\(^{st}\) edn (Doin, France, 2006)
Y.C. Wu, National Bureau of Standards (District of Columbia, Washington, 1982)
J.A. Weaver, A.M. Stoll, in NADC Memo Report 6708 (United Stated Naval Air Development Center, Johnsville, Pennsylvania, 1967)
D.C. Gaylor, Ph.D. thesis (Massachusetts Institute of Technology, USA, 1989)
J.A. Pearce, S.L. Thomsen, H. Vijverberg, T.J. McMurray, In Proceedings SPIE: Kinetics for birefringence changes in thermally coagulated rat skin collagen, vol. 1876, pp.180–185, (1993)
C.E. Fugitt, In Armed Forces Special Weapons Project AFSWP-606: A rate process of thermal injury (1955)
J. Whitton, J. Everall, Br. J. Derm. 89, 467 (1973)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sonneck-Museux, N., Scheer, E., Perez, L. et al. Development of a Skin Burn Predictive Model adapted to Laser Irradiation. Int J Thermophys 37, 122 (2016). https://doi.org/10.1007/s10765-016-2106-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-016-2106-5