Abstract
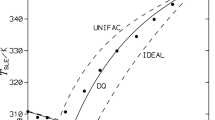
Solid-liquid equilibria for three binary mixtures of 2-nitrodiphenylamine (1) + diphenylamine (2), ethyl centralite (1) + N-ethyl-4-nitro-N-nitrosoaniline (2), and 2,2\(^{\prime }\)-dinitrodiphenylamine (1) + N-ethyl-4-nitro-N-nitrosoaniline (2) were measured using a differential scanning calorimeter. Simple eutectic behaviors for these systems were observed. The experimental results were correlated by means of original and modified NRTL, Wilson, and UNIQUAC equations. The root–mean–square deviations of the solubility temperatures for all measured data vary from 0.63 K to 3.73 K and depend on the particular model used. The best solubility correlation was obtained with the UNIQUAC model.












Similar content being viewed by others
References
H. Ritter, S. Braun, M. Kaiser, C. Becher, Propellants Explos. Pyrotech. 33, 203 (2008)
L.S. Lussier, E. Berger, H. Gapnon, Propellants Explos. Pyrotech. 31, 253 (2006)
L.S. Lussier, H. Gagnon, in Development of Modern Methods for Determination of Stabilizers in Propellants (Defense Research Establishment, Valcartier, Quebec, DREV R-9511, 1996)
A. Mekki, K. Khimeche, A. Dahmani, J. Chem. Thermodyn. 42, 1050 (2010)
K. Khimeche, A. Dahmani, J. Therm. Anal. Calorim. 84, 47 (2006)
K. Khimeche, A. Dahmani, J. Chem. Thermodyn. 38, 1192 (2006)
K. Khimeche, Y. Boumrah, M. Benziane, A. Dahmani, Thermochim. Acta 444, 165 (2006)
M. Mular, A. Vom Berg, Technical Memorandum WSRL-0294-TM (Weapons Systems Research Laboratory, Adelaide, South Australia, 1982)
N.J. Curtis, Technical Report WSRL-0563-TR (Weapons Systems Research Laboratory, Salisbury, South Australia, 1987)
M.S. Elliot, F.J. Smith, A.M. Fraser, Propellants Explos. Pyrotech. 25, 31 (2000)
N.J. Curtis, Technical Report WSRL-0436-TR (Weapons Systems Research Laboratory, Salisbury, South Australia, 1986)
V. Chevallier, D. Petitjean, V.R. Meray, M. Dirand, Polymer 40, 5953 (1999)
Y.P. Chen, M. Tang, J.C. Kuo, Fluid Phase Equilib. 232, 182 (2005)
D. Mackay, W.Y. Shiu, K. Ma, S.C. Lee, Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals, 2nd edn. (Taylor and Francis, New York, 2006)
R. Meyer, J. Köler, A. Homburg, Explosives, 6th edn. (Wiley, Weinheim, 2007)
E.J. Baum, Chemical Property Estimation: Theory and Application (CRC Press, Boca Raton, FL, 2000)
J. Quinchon, J. Tranchant, Les poudres, propergols et explosifs, vol. 2 (Technique et Documentation, Lavoisier, France, 1984)
D.R. Lide, CRC Handbook of Chemistry and Physics, 89th edn. (CRC Press, Boca Raton, FL, 2008)
W.E. Acree Jr, Thermochim. Acta 189, 37 (1991)
C.R. Witschonke, Anal. Chem. 26, 562 (1954)
U. Domanska, J.A. Gonzalez, Fluid Phase Equilib. 123, 167 (1996)
U. Domanska, J. Lachwa, J. Chem. Thermodyn. 37, 692 (2005)
R.N. Rai, U.S. Rai, Thermochim. Acta 363, 23 (2000)
J.M. Praunitz, R.N. Lichtenthaler, E.G. Azevedo, Molecular Thermodynamics of Fluid Phase Equilibria, 2nd edn. (Prentice-Hall, Englewood Cliffs, NJ, 1986)
J.A.P. Coutinho, S.I. Andersen, E.H. Stenby, Fluid Phase Equilib. 117, 138 (1996)
U. Domanska, F.R. Groves, E. Mc Laughlin, J. Chem. Eng. Data 38, 88 (1993)
C. Pan, M. Radosz, Fluid Phase Equilib. 155, 57 (1999)
R.V. Orye, J.M. Prausnitz, Ind. Eng. Chem. 57, 18 (1965)
M. Mukhopadhyay, K. Sahasranaman, Ind. Eng. Chem. Process Des. Dev. 21, 632 (1982)
J. Nagata, Y. Nakamiya, K. Katoh, J. Koyabu, Thermochim. Acta 45, 153 (1981)
J.A. Nelder, R. Mead, Comput. J. 7, 308 (1965)
T. Hofman, J. Nagata, Fluid Phase Equilib. 25, 113 (1986)
J.P. Monfort, M.D.L. Rojas, Fluid Phase Equilib. 2, 181 (1978)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trache, D., Khimeche, K. & Dahmani, A. Study of (Solid–Liquid) Phase Equilibria for Mixtures of Energetic Material Stabilizers and Prediction for Their Subsequent Performance. Int J Thermophys 34, 226–239 (2013). https://doi.org/10.1007/s10765-013-1404-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-013-1404-4