Abstract
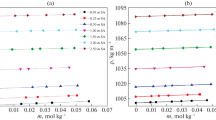
Viscosities of l-lysine monohydrochloride, l-histidine, and l-arginine in 1 m (mol · kg−1) aqueous solutions of sodium acetate, potassium acetate, and calcium acetate salts has been determined at (303.15, 308.15, 313.15, 318.15, and 323.15) K. The Falkenhagen coefficient, A, and Jones–Dole coefficient, B, relative viscosity, and specific viscosity of the solutions have also been determined using the measured viscosities. The results are interpreted in terms of solute–solute and solute–solvent interactions occurring in the system under investigation and also discussed in terms of the structure-making/breaking ability of the solute in these salt solutions. The structure making/breaking abilities of the solutes in the studied systems are strongly influenced by temperature.
Similar content being viewed by others
References
S.N. Timasheff, G.D. Fasman (eds.), Structure and Stability of Biological Macro Molecules, vol. II (Marcel Dekker, New York, 1969), pp. 65, 213
Badarayani R., Kumar A.: J. Chem. Eng. Data 48, 664 (2003)
Wadi R.K., Goyal R.K.: J. Solut. Chem. 21, 163 (1992)
Banipal T.S., Lark B.S., Patyar P., Kishore N.: J. Chem. Eng. Data 49, 553 (2004)
Singh S.K., Kishore N.: J. Solut. Chem. 32, 117 (2003)
Yan Z., Wang J., Jinsuo L.: J. Chem. Eng. Data 46, 217 (2001)
Wang J., Zhenning Y., Jinsuo L.: J. Chem. Thermodyn. 36, 281 (2004)
Banipal T.S., Damanjit K., Banipal P.K.: J. Chem. Eng. Data 49, 1236 (2004)
Belibagli K.B., Ayranci E.: J. Solut. Chem. 19, 867 (1990)
Yan Z., Wang J., Zheng H., Liu D.: J. Solut. Chem. 27, 473 (1998)
Wang J., Liu D., Lu J.: Z. Phys. Chem. 211, 121 (1999)
Herskovits T.T., San George R.C., Cavanagh S.M.: J. Colloid Interface Sci. 63, 226 (1978)
Robinson D.R., Jencks W.P.: J. Am. Chem. Soc. 87, 2470 (1965)
Siddique J.A., Naqvi S.: J. Chem. Eng. Data 55, 2930 (2010)
Siddique J.A., Naqvi S.: Chin. J. Chem. 29, 669 (2011)
Kestin J., Sokolov M., Wakeham W.A.: J. Phys. Chem. Ref. Data 7, 941 (1978)
Wadi R.K., Ramasami P.: J. Chem. Soc. Faraday Trans. 93, 243 (1997)
Bhat R., Ahluwalia J.C.: Int. J. Peptide Protein Res. 30, 145 (1987)
Dash U.N., Pasupalak N.N.: Indian J. Chem. 36, 834 (1997)
Jahagirdhar D.V., Arbad B.R., Patil S.C., Shankarwar A.G.: Indian J. Pure Appl. Phys. 38, 645 (2000)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siddique, J.A., Naqvi, S. Viscosity Behavior of α-Amino Acids in Acetate Salt Solutions at Temperatures (303.15 to 323.15) K. Int J Thermophys 33, 47–57 (2012). https://doi.org/10.1007/s10765-011-1111-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-011-1111-y