Abstract
Despite their importance to conservation strategies, baseline data on species distribution and abundance are lacking for many primates. Discovered 30 years ago, the blond titi (Callicebus barbarabrownae) is considered Critically Endangered. The first assessments in 2004-2006 sampled 126, 1-km radius areas using interviews, sightings, and playing back loud calls to confirm the presence of this species in 37 areas, suggesting a minimum population of 260 individuals in the Caatinga biome, Brazil. Ten years later, from 2015 to 2017, we reassessed the species’ distribution range using playback points to survey 224, 1-km radius areas, including 34 occurrence areas from 2004-2006. We counted the number of responding or sighted groups and individuals in each area. We also measured land cover change from 2005 to 2016 in the species’ geographic range. We recorded blond titis in 92 areas, including 28 of the 34 areas occupied in 2004-2006, and three protected areas. We found 194 groups and at least 404 individuals. From 2005 to 2016, forest cover decreased by 11% and urban area increased by 67%. The higher number of occupied areas and individuals we found in 2015-2017 are likely due to the higher sampling effort employed and higher detectability of playback surveys compared to interviews. Although the blond titi occurs in more areas and in higher numbers than previously known, threats related to land use are increasing. We suggest the use of improved observational (e.g., playback) and analytical methods to enhance knowledge of primate distribution and population size.
Similar content being viewed by others
Introduction
The distribution (geographic range size and area of occupancy) and abundance (local populations sizes and total number of individuals in the wild), plus dispersal ability strongly determine how prone a species is to extinction (Beissinger, 2000; Johnson, 1998). Thus, measures of distribution and abundance are key information for planning species conservation. Small range sizes, or low area of occupancy, or small populations can indicate high-priority species for protection (Habel et al., 2020; Mace et al., 2008). For instance, species action plans use the occupied areas and the size of local populations to indicate the most important areas for protection (IUCN/SSC, 2008; see primate action plans at http://www.primate-sg.org/action_plans). Distribution and abundance also are major variables to be monitored to evaluate the effectiveness of conservation actions (Stevenson et al., 2020). For example, increased abundance indicates that hunting bans (Wild et al., 2005), anti-poaching patrols (Aveling & Aveling, 1987), and creating/protecting habitat corridors (Horwich & Lyon, 1998) are likely beneficial actions for primate species (Junker, Kühl, et al., 2020a).
Despite its importance, distribution and abundance information are lacking for many primate species (Bezanson & McNamara, 2019; Estrada et al., 2017), contributing to the insufficient evidence for their effective conservation (Junker et al., 2020b). This is the case for the threatened blond titi (Callicebus barbarabrownae), inhabitant of the Caatinga biome in Brazil. Despite being described 30 years ago (Hershkovitz, 1990), this species still lacks such baseline information. The distribution and conservation status of the blond titi was first assessed in 2004-2005, indicating a 291,438 km2 extent of occurrence, an area of occupancy of 2,636 km2, and a minimum of 260 remaining individuals (Printes et al., 2011). Other studies presented new records in Bahia (in 2006, N = 5; Estrela et al., 2011) and Sergipe (between 2008 and 2010, N = 7; Marques et al., 2013) states. Before 2015, two studies estimated the size of local populations using sightings from line transects, and the few records suggested that the abundance of the species is low (Corsini & Moura, 2014; Freitas et al., 2011). However, in 2017, a population of 273 individuals was estimated by playback point counts in a 221-km2 area, and the authors highlighted the importance of using more effective detection methods for the species (Coelho et al., 2020). Studies so far indicate that the blond titi also occurs in short arboreal vegetation (Marques et al., 2013) but is mainly found in taller remnants of dry and evergreen forests in Caatinga, although it is not restricted to undisturbed forests (Corsini & Moura, 2014; Printes et al., 2011).
Given the information available, the blond titi has been assessed as Critically Endangered by the IUCN Red List from 1996 to 2015 (Printes et al., 2020) and by the Brazilian Official List of Threatened Species (Printes et al., 2018; Printes & Rylands, 2008). It was listed as one of the most endangered primates in the world from 2010 to 2012 (Mittermeier et al., 2012) and among the Evolutionarily Distinct and Globally Endangered (EDGE) species, which refers to threatened taxa that represent a significant amount of unique evolutionary history (ZSL, 2020). These conservation status assessments suggested habitat loss, degradation, and fragmentation as the main threats for the species. The blond titi also is hunted for meat, although it is probably not a preferential target (Beltrão-Mendes et al., unpublished data; Corsini & Moura, 2014). Considering its conservation status and the ever-increasing threats, the blond titi has gained increasing attention from the academy and government. In 2011, the National Action Plan for the Conservation of Northeastern Primates (NAP PRINE) instituted priority goals and actions for blond titi conservation (ICMBio, 2012), including obtaining baseline information on its distribution and abundance.
We aim to evaluate the distribution and minimum number of individuals of the blond titi in its known geographic range in 2015-2017. We surveyed 224, 1-km radius areas (703 km2) by playing back recordings of blond titi loud calls to count the number of responding or sighted groups and individuals. We resampled 34 occupied areas from the first assessments of this species in 2004-2006 to evaluate possible local extinctions. We measured and compared the habitat cover of the blond titi between 2005 and 2016 for its entire geographic range and locally in the past and current occupied areas.
Methods
Study region
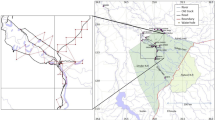
The known geographic range of the blond titi extends through 166,539 km2, mainly in the Caatinga biome, Northeastern Brazil (Fig. 1; Printes et al., 2018). Seasonally dry tropical forests and woodlands predominate in Caatinga, although floristic elements of savannas, tropical rain forests, and rupestrian grasslands are scantily represented in the region (Queiroz et al., 2017). The semiarid climate, characterized by irregular rainfall and extended droughts, results in deciduous trees and shrubs. The proportion of plants shedding their leaves during the dry season ranges from 70% to 100% in most vegetation patches (Queiroz et al., 2017). The current main vegetation types vary from open scrublands (cactus abundant) and medium-size woodlands to tall, dry forests (Queiroz et al., 2017; Silva et al., 2017). However, the name “Caatinga” literally means “white forest” in the indigenous Tupi language, and dry forests should be the dominant vegetation before human disturbance, as we can see from the isolated tall trees remaining in scrub and woodlands. Approximately 63% of the Caatinga biome has been modified by human activities, with 28.6 million people in 2015 living mainly in cities (64%) and showing the lowest human developments indicators in Brazil (Silva et al., 2017; Silva & Barbosa, 2017). Natural land cover conversion to commercial agriculture, pastures, and reservoirs, and the overexploitation of natural resources, such as collecting firewood and poaching, are likely to determine blond titi occurrence and abundance.
Areas sampled (each 1-km radius, 3.14 km2) using playback point counts in 2015-2017 for the occurrence of the blond titi (Callicebus barbarabrownae) in Northeastern Brazil. Map classification from Souza et al. (2020).
Playback point surveys
From August 2015 to December 2017, we conducted 6 field surveys in 80 days to perform 314 playback points across the geographic range of the blond titi, travelling 13,078 km in 53 municipalities of Bahia and Sergipe states. We surveyed in August–September 2015, September 2016, November–December 2016, and March, July–August, and December 2017, including rainy days and dry periods. Local people in the Caatinga report that blond titis call more often in rainy periods, suggesting that the species is more likely to respond to playback calls in rainy than dry periods. Because rain is for short and infrequent periods in Caatinga, we could not account for this possible bias. We chose playback sites based on the presence of vegetation patches potentially harboring blond titis and considering locations reported in the literature. We played back close (range: 2-2,400 m) to the sites where blond titis are reported in Printes et al. (2011) and Estrela et al. (2011), and in the same region (5-km away) as records in Marques et al. (2013) and Corsini and Moura (2014).
We used the 314 playback points to sample the occurrence of the blond titi in 224, 1-km radius areas, totaling 703 km2 (Fig. 1; Electronic Supplementary Material [ESM] Table SI). During fieldwork, we noticed that blond titi responses to playback can come from 1-km away, although most records were up to 500-m away. To obtain meaningful observed areas that we could compare with previous studies, we grouped playback points within 1 km of each other in the same sample area (1-km radius, 3.14 km2). The distance between playback points varied from 35 m to 40.5 km, so we sampled 58, 1-km radius areas by more than one playback point (from 2-7; ESM Table SI).
We surveyed each playback point once, by playing a 1.5-min call three times followed by a 4-min listening period (Coelho et al., 2020). Using a Megaphone SK66 25 W in all cardinal directions, we played a duet loud call recording, performed by free-ranging blond titi, around 100 dB measured at 1 m. To determine the minimum abundance of groups and individuals at each playback point, two observers independently recorded the number of groups and individuals based on vocal responses or sightings. We located different responding groups using call directions agreed by the two observers. We recorded the minimum number of responding individuals per group as one or two by distinguishing solo and duet calls (Adret et al., 2018). We were not confident in differentiating more than two calling individuals in the same group. We determined the total number of blond titis recorded at each playback point based as the higher number of sighted or heard individuals or by the sum when we were sure we sighted and heard different individuals. Considering the small home ranges of titi species (the largest home range is estimated in 0.48 km2; Bicca-Marques & Heymann, 2013) and the locations that we estimated for each blond titi group, we are confident we did not count the same group or individuals at different playback points. We recorded the vegetation type for each group recorded, except at two playback points (3 groups) where we could not define the approximate group location well (ESM Table SI).
To evaluate possible local extinctions in 10 years, we resampled 34 of 37 areas occupied in the first assessments of the species. We grouped locations sampled in 2004-2006 (data from Estrela et al., 2011 and Printes et al., 2011) using the same criterion that locations less than 1-km apart represent the same 1-km radius sampled area. In 2004-2006, the occurrence of blond titi was sampled in 126, 1-km radius (395 km2) by interviews, and positive responses (N = 37 areas) were confirmed by sightings or playback responses (Estrela et al., 2011; Printes et al., 2011).
Land cover change over 10 years
Blond titi abundance is related to habitat amount and distance from human settlements (Coelho et al., 2020). We measured the amount of blond titi potential habitat and urban areas in 2005 and 2016, for the whole geographic range of the species and in each 1-km radius occupied area. We used the land use and land cover maps (30-m × 30-m pixel resolution) produced by MapBiomas (Souza et al., 2020). Based on field observations of the vegetation, we identified two land cover classes defined in MapBiomas as potential habitat for the blond titi: Forest and Savanna. These classes include the five local vegetation types probably inhabited by blond titis: (i) riverine forests, (ii) high-altitude forests (both evergreen arboreal vegetation), (iii) short (up to 15 m), and (iv) tall (15-30 m) dry forests (deciduous or semideciduous arboreal vegetation), and (v) woodlands (dense tall shrubs sometimes mixed with palms, cactus, and medium-sized trees). Forest includes only patches of short and tall dry forests, riverine and high-altitude forests (Fig. 2). Savanna, however, must be interpreted carefully as blond titi habitat, because it includes vegetation probably not inhabited by blond titis. We found land cover classified as Savanna that ranged from sparse short shrubs in pastures or bare ground, including a few trees in places (probably not blond titi habitat, but which they may use to move between habitat patches), to vegetation probably inhabited by the blond titi, such as woodlands, short, and tall dry forests (Fig. 3). We used QGIS (QGIS Development Team, 2018) and the “landscapemetrics” package (Hesselbarth et al., 2019) in the R v3.6.3 software environment (R Core Team, 2020) to measure the amount of Forest, Savanna, and Urban classes in the entire geographic range of the blond titi and for each 1-km radius area (3.14 km2) where blond titi occurred in 2004-2006 or 2015-2017. We converted map classifications to the Albers Equal Area Conic coordinate reference system to measure areas.
Vegetation types in the geographic range of blond titis (Callicebus barbarabrownae), Northeastern Brazil, identified as Forest class by MapBiomas land use and land cover classification (Souza et al., 2020). A Short, dry forest (Miguel Calmon, BA; Lat −11.4725°, Long −40.6932°), (B) tall dry forest (Orobó Area of Relevant Ecological Interest, Itaberaba, BA; Lat −12.3138°, Long −40.4711°; red circle shows two blond titis), and (C) riverine forest (Chapada Diamantina National Park, Andaraí, BA; Lat −12.6833°, Long −41.3345°). Datum WGS 84.
Vegetation types in the geographic range of blond titis (Callicebus barbarabrownae), Northeastern Brazil, identified as Savanna class by MapBiomas land use and land cover classification (Souza et al., 2020). A Sparse short shrubs (Boa Vista do Tupim, BA; Lat −40.6155, Long −12.5111), (B) woodlands (Gentio do Ouro, BA; Lat −11.3867°, Long −42.3669°), (C) short, dry forest (Jeremoabo, BA; Lat −9.9720°, Long −38.2441°), and (D) tall dry forest (Itiúba, BA; Lat −10.6970°, Long −39.8291°; red circles show four blond titis).
Ethical note
We comply with the Code of Best Practices for Field Primatology, and this study is authorized by Chico Mendes Brazilian Institute for Biodiversity Conservation (SISBIO 52222-1). The research adhered to the American Society of Primatologists Principles for the Ethical Treatment of Non-Human Primates. The authors declare that they have no conflict of interest
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Results
We recorded the occurrence of the blond titi in 92 (41%) of the 224, 1-km radius areas we sampled in 2015-2017, including 29 municipalities in Bahia state and 2 in Sergipe state (Fig. 1; Table I). We did not detect the species in 132 areas (Fig. 1; ESM Table SI). We confirmed the species occurrence in three protected areas (Orobó Area of Relevant Ecological Interest, Chapada Diamantina National Park, and Marimbus-Iraquara Environmental Protection Area) and in the immediate surroundings (1 km) of Contendas do Sincorá National Forest. Comparing the occurrence areas of the blond titi with the first assessments, we found the species in 28 of 34 areas it occurred in 2004-2006 and in another 64 areas not sampled previously (Table I). These includes a new western limit for the species (Latitude −11.384070 °, Longitude 42.702991 °; Datum WGS84) in Itajubaquara (Gentio do Ouro, BA), around 35 km west of the previous known limit.
We detected blond titis by their vocalizations in response to playback calls, with 1 to 12 individuals (6 groups) in a single area (Table I; ESM Table SI). In 22 areas, we also saw a total of 66 individuals in 24 groups (ESM Table SI; ESM Table SII). Overall, we recorded 192 different groups and 400 individuals in the areas we sampled using playback. In addition, we recorded two groups (2 individuals each) by their calls in Marimbus-Iraquara Environmental Protection Area (we recorded another group during the playback session in the same area, ID 204). The 404 recorded individuals can be considered the minimum number of blond titis living in the species’ geographic range. We recorded most groups were in short (N = 104 groups) and tall (N = 71) dry forests, but also found blond titis in riverine forests (N = 6), high-altitude forests (N = 4), and woodlands (N = 4; ESM Table SI). We did not detect the blond titi in sparse shrubs.
Potential blond titi habitat decreased from 2005 to 2016 (Forest and Savanna) and human occupation increased (Urban) in the species’ geographic range of 166,539 km2. Forest cover decreased by 11% (range: 7,577 km2 – 6,719 km2), but Savana decreased only by 2% (range: 72,096 km2 – 70,162 km2). Urban increased by 67% in the region, from 178 km2 in 2005 to 297 km2 in 2016. Considering only the 98 areas (3.14 km2 each) where the blond titi occurred in 2004-2006 or in 2015-2017 (Table I), Forest also decreased (by 6.6%, from 42.58 km2 to 39.75 km2), and Savanna decreased by a very small amount (by 0.14%; Table I) after 10 years. There was no Urban class in these areas in either period, although there are small settlements in some areas. The 92 occurrence areas in 2015-2017 showed a mean of 1.9 km2 (± SD 0.78) of Forest and Savanna in 2016, with a minimum of 0.19 km2 and a maximum of 3.14 km2 (Table I). In the six areas where the blond titi occurred in 2004-2006 but was not detected in 2015-2017, there was an 11% reduction of potential habitat (Table I).
Discussion
Our reassessment of the distribution of the blond titi, 10 years after its first evaluation, revealed a higher number of occurrence areas and individuals than previously known. Studies in 2004-2006 (Estrela et al., 2011; Printes et al., 2011) recorded the blond titi in 37 areas (29% of 126 areas sampled by interviews). Our findings of the species in 92 areas (40% of 224 areas sampled by playback points) represented an increase of 148% in the known number of occupied areas. The minimum number of living individuals in the wild was (n = 404) 1.5 times the previous estimate of 260 individuals.
The difference observed between 2004-2006 and 2015-2017 assessments does not mean that blond titi occurrence and abundance increased in 10 years. It can be explained both by the higher sampling effort that we employed and the higher detectability of the method we used. We surveyed 98 more areas than in 2004-2006, a region 77% larger (703 km2 vs. 395 km2). This increased sampled area partly explains the higher number of occupied areas. Moreover, titi monkeys (subfamily Callicebinae, Byrne et al., 2016) are hard to detect, because they are shy and cryptic, live in small groups, and move short distances daily (mean 600-700 m) through small home ranges (range: 0.01-0.48 km2; Bicca-Marques & Heymann, 2013). The active acoustic method (playback) that we applied is more effective than interviews or visual search in detecting the presence and counting groups of titi monkeys (Coelho et al., 2020; Corsini & Moura, 2014; Dacier et al., 2011; Gestich et al., 2016). Still, playback detection is only moderate (0.48 in surveys from sunrise to mid-morning) to low (0.11 in surveys from mid-morning to sunset) and can vary between seasons and sites (Coelho et al., 2020). We conducted only 16% of our playback surveys from sunrise to mid-morning (10:00 h; ESM Table SI). Considering the detectability of interviews and playback, some areas with no detection in 2004-2006 (89) or in 2015-2017 (132) may be inhabited by the blond titi.
Besides the higher sampling effort and detectability in 2015-2017, a true increase in occurrence areas and abundance of the blond titi in 10 years is very unlikely because of the land cover change in its geographic range during this period. Potential habitat (Forest and Savanna classes) decreased by 3.5%, and Forest alone decreased by 11%. At the same time, urban human occupation increased by 67%, accompanying human population growth, probably resulting in increased hunting and exploitation of natural vegetation. Moreover, it is possible that the species is now locally extinct in six areas occupied in 2004-2006 but with no detection in 2015-2017, where we recorded burning, selective logging, deforestation, and mining activity during surveys. Forest and Savanna decreased in four of these areas (from 11% in area ID35 to 38% in area ID136), although higher decrease occurred in 8 of 28 areas where we still found blond titis (from 0.4% in area ID112 to 50% in area ID145; Table I).
We found blond titis inhabiting different types of forests (short and tall dry forests, riverine, and high-altitude) and present the first four records of the species in woodlands (ESM Table SI). Other Callicebinae species occur in woodlands or bushy forests (Bicca-Marques & Heymann, 2013). As in a previous study (Printes et al., 2011), we mainly found blond titis in short dry forests, although we also sampled this vegetation more. Another study suggests blond titis prefer taller and humid forests (Corsini & Moura, 2014). Our data suggest that the species is a forest-dweller but may survive in remnant vegetation that was previously forest (like most woodlands in Caatinga), because Callicebinae species survive even in disturbed habitats, such as secondary forests (Bicca-Marques & Heymann, 2013; Pinto et al., 1993).
Although our results represent the best baseline information available for the species so far, our data have some limitations that should be considered in the design of future studies on the occurrence and abundance of primates. Presence-absence data like ours are limited in informing the area of occupancy (actual area or number of sites occupied by a species) and tracking its change over time, unless detectability is constant across space and time (Araújo et al., 2019; Guillera-Arroita et al., 2015), a rare situation that clearly is not the case in our study. Our data are limited in estimating abundance from the responding groups that we counted, because our single survey at each playback point lacks information about the detection process, which can be obtained by temporal replicates, multiple observers (dependent or independent), multiple survey methods, site spatial subsampling, or time to detection protocols (Dénes et al., 2015; Guillera-Arroita, 2017). Considering the effect of low detectability on our data, it is highly probable that the species does occur in other areas, including some areas surveyed here, and the actual number of individuals may be much higher than our counts. The only study so far using temporal replicates of playback surveys and analyses dealing with imperfect detection found a local population of 271 adult blond titis (2.3 individuals/km2) in the dry forests of Boa Vista do Tupim (Coelho et al., 2020).
The blond titi has been evaluated as Critically Endangered by the IUCN Red List (last evaluation in 2015; Printes et al., 2020) and in Brazil (last evaluation in 2015; Printes et al., 2018). This category was assigned by the IUCN Red List from 1996 to 2008 based on the very few sites (<5) where the species was known to occur. The new sites recorded in the 2015 evaluation expand the known range, and the criterion adopted changed to remnant population sizes, C2a(i): the population size is estimated to number fewer than 250 adult individuals, the population is experiencing a continuous decline, and no subpopulation is estimated to contain more than 50 adult individuals (IUCN, 2012). Our findings show that criteria C2a(i) does not apply for the blond titi, implying the need to review its conservation status. Moreover, the previously suggested rarity of the species (Corsini & Moura, 2014; Freitas et al., 2011; Printes et al., 2011) is not confirmed and is most likely related to sampling effort and detectability of the survey methods used.
In contrast, our results show increasing threats to blond titi conservation. Little forest cover remains in its geographic range (4.5%), the potential habitat (Forest and Savanna) available covers less than half the region (47.8%), and only 1% is currently protected areas. The observed decrease in potential habitats, including in blond titi occurrence areas, and increase in human occupation are of special concern considering that only 7.4% of the Caatinga biome is protected areas, and 94% of the region has moderate to high desertification risk (Vieira et al., 2015). Climate and land use changes affect the projected distribution of Callicebus species and will lead to a worrisome scenario if environmental degradation continues (Gouveia et al., 2016). As urgent measures to face this trend, we highlight the need to assure a viable population of the species in strictly protected areas and the restoration of forests to increase the quality, size, and connectivity of the available habitat.
Our study expands information on the Endangered blond titi, providing background for the species’ action plans (ICMBio, 2018). Further studies, using improved observational (e.g., playback surveys) and analytical methods, are needed to enhance our knowledge about distribution, abundance, and habitat use of blond titi populations, and for many other Endangered primate species (Estrada et al., 2017). Adopting appropriate study designs (Smith et al., 2017; Thompson, 2012; Williams & Brown, 2019), survey methods (aiming for higher detection, e.g., playback sampling for responsive primates) and survey protocols (data informative on the detection process) appropriate for the target species are crucial for accurate analyses (Elphick, 2008; Guillera-Arroita, 2017; Murray & Sandercock, 2020) to achieve the much-needed knowledge of primate distribution and abundance and changes in these variables. Only by using consistent data from intense monitoring over long periods can we rigorously evaluate the effects of conservation interventions on primates (Junker et al., 2020a, b) and improve species’ action plans.
References
Adret, P., Dingess, K., Caselli, C., Vermeer, J., Martínez, J., Amancio, J. C. L., … & Di Fiore, A. (2018). Duetting patterns of titi monkeys (Primates, Pitheciidae: Callicebinae) and relationships with phylogeny. Animals, 8, 178.
Araújo, M. B., Anderson, R. P., Márcia Barbosa, A., Beale, C. M., Dormann, C. F., Early, R., … & Rahbek, C. (2019). Standards for distribution models in biodiversity assessments. Science Advances 5, eaat4858.
Aveling, R., & Aveling, C. (1987). Report from the Zaire Gorilla Conservation Project. Primate Conservation, 8, 162–164.
Beissinger, S. R. (2000). Ecological mechanisms of extinction. Proceedings of the National Academy of Sciences of the United States of America, 97, 11688–11689.
Bezanson, M., & McNamara, A. (2019). The what and where of primate field research may be failing primate conservation. Evolutionary Anthropology: Issues, News, and Reviews, 28, 166–178. https://doi.org/10.1002/evan.21790.
Bicca-Marques, J. C., & Heymann, E. H. (2013). Ecology and behaviour of titi monkeys (genus Callicebus). In L. M. Veiga, A. Barnett, S. F. Ferrari, & M. A. Norconk (Eds.), Evolutionary biology and conservation of titis, sakis, and uacaris (pp. 196–207). Cambridge University Press.
Byrne, H., Rylands, A. B., Carneiro, J. C., Alfaro, J. W. L., Bertuol, F., Silva, M. N. F., ... & Boubli, J. P. (2016). Phylogenetic relationships of the New World titi monkeys (Callicebus): first appraisal of taxonomy based on molecular evidence. Frontiers in Zoology, 13, 10. https://doi.org/10.1186/s12983-016-0142-4
Coelho, I. P., Collins, S. J., Santos Junior, E. M., Valença-Montenegro, M. M., Jerusalinsky, L., & Alonso, A. C. (2020). Playbackpoint counts and N-mixture models suggest higher than expected abundance of the critically endangered blond titi monkey in Northeastern Brazil. American Journal of Primatology, 82(5), e23126. https://doi.org/10.1002/ajp.23126.
Corsini, C. F., & Moura, A. C. A. (2014). Census of the blond titi monkey Callicebus barbarabrownae (Pitheciidae) in the semi-deciduous Atlantic forest of Chapada Diamantina, Brazil. Neotroprical Primates, 21, 177–182. https://doi.org/10.1896/044.021.0203.
Dacier, A., Luna, A. G., Fernandez-Duque, E., & Di Fiore, A. (2011). Estimating population density of Amazonian titi monkeys (Callicebus discolor) via playback point counts. Biotropica, 43, 135–140. https://doi.org/10.1111/j.1744-7429.2010.00749.x.
Dénes, F. V., Silveira, L. F., & Beissinger, S. R. (2015). Estimating abundance of unmarked animal populations: accounting for imperfect detection and other sources of zero inflation. Methods in Ecology and Evolution, 6, 543–556. https://doi.org/10.1111/2041-210X.12333.
Elphick, C. S. (2008). How you count counts: the importance of methods research in applied ecology. Journal of Applied Ecology, 45, 1313–1320. https://doi.org/10.1111/j.1365-2664.2008.01545.x.
Estrada, A., Garber, P. A., Rylands, A. B., Roos, C., Fernandez-Duque, E., Di Fiore, A., ... & Li, B. (2017). Impending extinction crisis of the world’s primates: why primates matter. Science Advances, 3, e1600946. https://doi.org/10.1126/sciadv.1600946
Estrela, A. R., Nogueira, E. M. S., & Porfírio, S. (2011). Callicebus barbarabrownae (Hershkovitz, 1990) (Primates: Pitheciidae) de Lamarão/BA: resultados preliminares. In F. R. Melo & I. Mourthé (Eds.), A Primatologia no Brasil (Vol. 11, pp. 93–102). Sociedade Brasileira de Primatologia.
Freitas, E. B., De-Carvalho, C. B., & Ferrari, S. F. (2011). Abundance of Callicebus barbarabrownae (Hershkovitz 1990), (Primates: Pitheciidae) and other nonvolant mammals in a fragment of arboreal Caatinga in Northeastern Brazil. Mammalia, 75, 339–343. https://doi.org/10.1515/MAMM.2011.047.
Gestich, C. C., Caselly, C. B., Nagy-Reis, M. B., Setz, E. Z. F., & Cunha, R. G. T. (2016). Estimating primate population densities: the systematic use of playbacks along transects in population surveys. American Journal of Primatology, 9999, 1–9. https://doi.org/10.1002/ajp.22586.
Gouveia, S. F., Souza-Alves, J. P., Rattis, L., Dobrovolski, R., Jerusalinsky, L., Beltrão-Mendes, R., & Ferrari, S. F. (2016). Climate and land use changes will degrade the configuration of the landscape for titi monkeys in eastern Brazil. Global Change Biology, 22(6), 2003–2012.
Guillera-Arroita, G. (2017). Modelling of species distributions, range dynamics and communities under imperfect detection: advances, challenges and opportunities. Ecography, 40, 281–295. https://doi.org/10.1111/ecog.02445.
Guillera-Arroita, G., Lahoz-Monfort, J. J., Elith, J., Gordon, A., Kujala, H., Lentini, P. E., … & Wintle, B. A. (2015). Is my species distribution model fit for purpose? Matching data and models to applications. Global Ecology and Biogeography, 24, 276–292.
Habel, J. C., Gossner, M. M., & Schmitt, T. (2020). What makes a species a priority for nature conservation? Animal Conservation, 23, 28–35. https://doi.org/10.1111/acv.12512.
Hershkovitz, P. (1990). Titis, new world monkeys of the genus Callicebus (Cebidae, Platyrrhini): a preliminary taxonomic review. Fieldiana Zoology, 5, 1–109. https://doi.org/10.5962/bhl.title.2843.
Hesselbarth, M. H. K., Sciaini, M., With, K. A., Wiegand, K., & Nowosad, J. (2019). Landscapemetrics: an open-source R tool to calculate landscape metrics. Ecography, 42, 1648–1657.
Horwich, R. H., & Lyon, J. (1998). Community-based development as a conservation tool: The Community Baboon Sanctuary and the Gales Point, Manatee project. In R. B. Primack, D. Bray, H. A. Galletti, & I. Ponciano (Eds.), Timber, tourists, and temples: conservation and development in the Maya Forest of Belize, Guatemala and Mexico. Island Press.
ICMBio. (2012). Plano de Ação Nacional para a Conservação dos Primatas do Nordeste -PAN Primatas do Nordeste, Pub. L. No. Portaria n.o 37, § 1, Diário Oficial da União 73.
ICMBio. (2018). Plano de Ação Nacional para a Conservação dos Primatas do Nordeste -PAN Primatas do Nordeste - 2o. Ciclo, Pub. L. No. Portaria n° 242, § 1, Diário Oficial da União 61.
IUCN (2012). IUCN Red List Categories and Criteria: Version 3.1 ((2nd ed.) ed.) https://portals.iucn.org/library/node/10315.
IUCN/SSC. (2008). Strategic Planning for Species Conservation: A Handbook. Version 1.0. Gland, Switzerland: IUCN Species Survival Commission. 104 pp. https://www.iucn.org/downloads/scshandbook_2_12_08_compressed.pdf
Johnson, C. N. (1998). Species extinction and the relationship between distribution and abundance. Nature, 394, 272–274.
Junker, J., Kühl, H. S., Orth, L., Smith, R. K., Petrovan, S. O., & Sutherland, W. J. (2020a). Primate Conservation. In W. J. Sutherland, L. V. Dicks, S. O. Petrovan, & R. K. Smith (Eds.), What Works in Conservation 2020 (pp. 431–482). Open Book Publishers.
Junker, J., Petrovan, S. O., Arroyo-Rodríguez, V., Boonratana, R., Byler, D., et al (2020b). A severe lack of evidence limits effective conservation of the world's primates. BioScience, 70, 794–803. https://doi.org/10.1093/biosci/biaa082.
Mace, G. M., N. J. Collar, K. J. Gaston, C. Hilton-Taylor, H. R. Akçakaya, N. Leader-Williams, … & Stuart, S. N. (2008). Quantification of extinction risk: IUCN's system for classifying threatened species. Conservation Biology, 22, 1424–1442. https://doi.org/10.1111/j.1523-1739.2008.01044.x
Marques, E. L. N., Beltrão-Mendes, R., & Ferrari, S. F. (2013). Primates, Pitheciidae, Callicebus barbarabrownae Hershkovitz, 1990: New localities for the critically endangered titi monkey in the São Francisco basin, state of Sergipe, Brazil. Check List, 9, 113–115. https://doi.org/10.15560/9.1.113.
Mittermeier, R. A., Rylands, A. B., Schwitzer, C., Taylor, L. A., Chiozza, F., & Williamson, E. A. (2012). Primates in Peril: The World’s 25 Most Endangered Primates 2010–2012. IUCN/SSC Primate Specialist Group (PSG), International Primatological Society (IPS), and Conservation International (CI). Arlington, VA. 40 pp.
Murray, D. L., & Sandercock, B. K. (2020). Population ecology in practice. Wiley.
Pinto, L. P. S., Costa, C. M., Strier, K. B., & da Fonseca, G. A. (1993). Habitat, density and group size of primates in a Brazilian tropical forest. Folia Primatologica, 61(3), 135–143.
Printes, R. C., & Rylands, A. B. (2008). Callicebus barbarabrownae (Hershkovitz, 1990). In A. B. M. Machado, G. M. Drummond, & A. P. Paglia (Eds.), Livro Vermelho da Fauna Brasileira Ameaçada de Extinção - Volume II (pp. 764–766). Ministério do Meio Ambiente e Fundação Biodiversitas.
Printes, R. C., Rylands, A. B., & Bicca-Marques, J. C. (2011). Distribution and status of the Critically Endangered blond titi monkey Callicebus barbarabrownae of north-east Brazil. Oryx, 45, 439–443. https://doi.org/10.1017/S0030605311000111.
Printes, R. C., Alonso, A. C., & Jerusalinsky, L. (2018). Callicebus barbarabrownae Hershkovitz, 1991. In ICMBio/MMA, Livro Vermelho da Fauna Brasileira Ameaçada de Extinção: Volume II – Mamíferos (pp. 283-287). Brasília, Brasil.
Printes, R., Alonso, A. C., Jerusalinsky, L., & Mittermeier, R. A. (2020). Callicebus barbarabrownae. The IUCN Red List of Threatened Species 2020: e.T39929A17974834. https://doi.org/10.2305/IUCN.UK.2020-2.RLTS.T39929A17974834.en.
QGIS Development Team (2018). QGIS Geographic Information System. Open Source Geospatial Foundation Project http://www.qgis.org/.
Queiroz, L. P., Cardoso, D., Fernandes, M. F., & Moro, M. F. (2017). Diversity and evolution of flowering plants of the caatinga domain. In J. M. C. Silva, I. R. Leal, & M. Tabarelli (Eds.), Caatinga: the largest tropical dry forest region in South America (pp. 23–63). Springer.
R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing https://www.R-project.org/.
Silva, J. M. C., & Barbosa, L. C. F. (2017). Impact of human activities in the Caatinga. In J. M. C. Silva, I. R. Leal, & M. Tabarelli (Eds.), Caatinga: the largest tropical dry forest region in South America (pp. 359–368). Springer.
Silva, J. M. C., Barbosa, L. C. F., Leal, I. R., & Tabarelli, M. (2017). The Caatinga: understanding the challenges. In J. M. C. Silva, I. R. Leal, & M. Tabarelli (Eds.), Caatinga: the largest tropical dry forest region in South America (pp. 3–19). Springer.
Smith, A. N. H., Anderson, M. J., & Pawley, M. D. M. (2017). Could ecologists be more random? Straightforward alternatives to haphazard spatial sampling. Ecography, 40, 1251–1255. https://doi.org/10.1111/ecog.02821.
Souza, C. M. Z., Shimbo, J., Rosa, M. R., Parente, L. L. A., Alencar, A., Rudorff, B. F. T., ... & Azevedo, T. (2020). Reconstructing three decades of land use and land cover changes in Brazilian biomes with Landsat archive and Earth Engine. Remote Sensing, 12, 2735. https://doi.org/10.3390/rs12172735
Stevenson, S. L., Watermeyer, K., Caggiano, G., Fulton, E. A., Ferrier, S., & Nicholson, E. (2020). Matching biodiversity indicators to policy needs. Conservation Biology, 35, 522–532. https://doi.org/10.1111/cobi.13575.
Thompson, S. K. (2012). Sampling. Wiley.
Vieira, R. M. S. P, Tomasella, J., Alvalá, R. C. S., Sestini, M. F., Affonso, A. G., Rodriguez, D. A., ... & Santana, M. O. (2015). Identifying areas susceptible to desertification in the Brazilian Northeast. Solid Earth, 6, 347–360.
Wild, C., Morgan, B. J., & Dixson, A. (2005). Conservation of drill populations in Bakossiland, Cameroon: historical trends and current status. International Journal of Primatology, 26, 759–773.
Williams, B. K., & Brown, E. D. (2019). Sampling and analysis frameworks for inference in ecology. Methods in Ecology and Evolution, 10, 1832–1842. https://doi.org/10.1111/2041-210X.13279.
ZSL (2020). EDGE of Extinction Programme – Evolutionarily Distinct and Globally Endangered. Zoological Society of London http://wwwedgeofexistence.org.
Acknowledgments
The authors thank the field support from Gustavo Medeiros Netto (Morro de Pedra Farm), Antônio Estrela, Felipe Ferreira, and José Manoel Zelis Pereira. This study was financed by Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio), The Mohamed bin Zayed Species Conservation Fund (Project No. 162513903), and Zoological Society for the Conservation of Species and Populations (ZGAP, through NGO PriMatas). A. C. Alonso received fellowships SET-F (350639/2015-9) and DTI-B (380559/2018-8) from ICMBio and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). R. Beltrão-Mendes received fellowship (88887.320996/2019-00) from the Brazilian Coordination for Higher Education Personnel Training (CAPES) and was supported by The Mohamed bin Zayed Species Conservation Fund (12055114), Primate Action Fund (1001257), and Primate Conservation Inc. (1158). The authors thank two anonymous reviewers, João Pedro Souza-Alves and Joanna M. Setchell, for the input on previous drafts of the manuscript.
Author information
Authors and Affiliations
Contributions
LJ, MMVM, ACA, and EM conceptualized the study. LJ, MMVM, ACA, and RBM obtained financial resources. ACA led the fieldwork and data collection, with the input of EM, RMB, and IPC. IPC conducted the analysis, assisted by ACA. IPC and ACA wrote the first draft and EM, MMVM, RBM, RCP, and LJ revised the manuscript and approved the final version.
Corresponding author
Additional information
Handling Editor: Joanna Setchell
Rights and permissions
About this article
Cite this article
Alonso, A.C., Coelho, I.P., Marques, E. et al. On the occurrence of the Critically Endangered blond titi (Callicebus barbarabrownae): reassessment of occupied areas and minimum population size. Int J Primatol 45, 35–53 (2024). https://doi.org/10.1007/s10764-021-00269-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-021-00269-5