Abstract
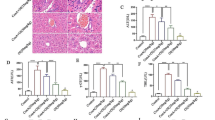
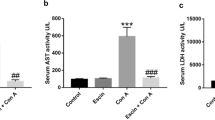
Autoimmune hepatitis (AIH) is a severe immune-mediated inflammatory liver disease that currently lacks feasible drug treatment methods. Our study aimed to evaluate the protective effect of succinic acid against AIH and provide a reliable method for the clinical treatment of AIH. We performed an in vivo study of the effects of succinic acid on concanavalin A (ConA)-induced liver injury in mice. We examined liver transaminase levels, performed hematoxylin and eosin (HE) staining, and observed apoptotic phenotypes in mice. We performed flow cytometry to detect changes in the number of neutrophils and monocytes, and used liposomes to eliminate the liver Kupffer cells and evaluate their role. We performed bioinformatics analysis, reverse transcription-quantitative polymerase chain reaction (RT-qPCR), and western blotting to detect mitochondrial apoptosis-induced changes in proteins from the B-cell lymphoma 2(Bcl-2) family. Succinic acid ameliorated ConA-induced AIH in a concentration-dependent manner, as reflected in the survival curve. HE and TUNEL staining and terminal deoxynucleotidyl transferase dUTP nick end labeling revealed decreased alanine transaminase and aspartate aminotransferase levels, and reduced liver inflammation and apoptosis. RT-qPCR and enzyme-linked immunosorbent assay revealed that succinic acid significantly reduced liver pro-inflammatory cytokine levels. Flow cytometry revealed significantly decreased levels of liver neutrophils. Moreover, the protective effect of succinic acid disappeared after the Kupffer cells were eliminated, confirming their important role in the effect. Bioinformatics analysis, RT-qPCR, and western blotting showed that succinic acid-induced changes in proteins from the Bcl-2 family involved mitochondrial apoptosis, indicating the molecular mechanism underlying the protective effect of succinic acid. Succinic acid ameliorated ConA-induced liver injury by regulating immune balance, inhibiting pro-inflammatory factors, and promoting anti-apoptotic proteins in the liver. This study provides novel insights into the biological functions and therapeutic potential of succinic acid in the treatment of autoimmune liver injury.





Similar content being viewed by others
Availability of Data and Materials
The datasets supporting the conclusions of this article are included within the article and its supplementary information files.
References
Goel, A., and P. Kwo. 2024. Treatment of autoimmune hepatitis. Clinics in Liver Disease 28 (1): 51–61.
Beretta-Piccoli, B.T., G. Mieli-Vergani, and D. Vergani. 2022. Autoimmmune hepatitis. Cellular & Molecular Immunology 19 (2): 158–176.
Pabst, O., M.W. Hornef, F.G. Schaap, V. Cerovic, T. Clavel, and T. Bruns. 2023. Gut-liver axis: Barriers and functional circuits. Nature Reviews Gastroenterology & Hepatology 20 (7): 447–461.
Tilg, H., T.E. Adolph, and M. Trauner. 2022. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metabolism 34 (11): 1700–1718.
Sass, G., S. Heinlein, A. Agli, R. Bang, J. Schümann, and G. Tiegs. 2002. Cytokine expression in three mouse models of experimental hepatitis. Cytokine 19 (3): 115–120.
Khan, H.A., M.Z. Ahmad, J.A. Khan, and M.I. Arshad. 2017. Crosstalk of liver immune cells and cell death mechanisms in different murine models of liver injury and its clinical relevance. Hepatobiliary & Pancreatic Diseases International 16 (3): 245–256.
Fujita, T., K. Soontrapa, Y. Ito, K. Iwaisako, C.S. Moniaga, M. Asagiri, et al. 2016. Hepatic stellate cells relay inflammation signaling from sinusoids to parenchyma in mouse models of immune-mediated hepatitis. Hepatology 63 (4): 1325–1339.
Wei, Y.-h, X. Ma, J.-C. Zhao, X.-Q. Wang, and C.-Q. Gao. 2023. Succinate metabolism and its regulation of host-microbe interactions. Gut Microbes. 15 (1).
Yuan, Y., Y. Xu, J. Xu, B. Liang, X. Cai, C. Zhu, et al. 2017. Succinate promotes skeletal muscle protein synthesis via Erk1/2 signaling pathway. Molecular Medicine Reports. 16 (5): 7361–7366.
Wang, K., M. Liao, N. Zhou, L. Bao, K. Ma, Z. Zheng, et al. 2019. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Reports 26 (1): 222.
Moyon, A., P. Garrigue, L. Balasse, S. Fernandez, P. Brige, A. Bouhlel, et al. 2021. Succinate injection rescues vasculature and improves functional recovery following acute peripheral ischemia in rodents: A multimodal imaging study. Cells 10 (4).
Cao, Z., S. Mu, M. Wang, Y. Zhang, G. Zou, X. Yuan, et al. 2023. Succinate pretreatment attenuates intestinal ischemia-reperfusion injury by inhibiting necroptosis and inflammation via upregulating Klf4. International Immunopharmacology 120.
Nadjsombati, M.S., J.W. McGinty, M.R. Lyons-Cohen, J.B. Jaffe, L. DiPeso, C. Schneider, et al. 2018. Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity 49 (1): 33.
Schneider, C., C.E. O’Leary, J. von Moltke, H.-E. Liang, Q.Y. Ang, P.J. Turnbaugh, et al. 2018. A metabolite-triggered tuft cell-ILC2 circuit drives small intestinal remodeling. Cell 174 (2): 271.
Macias-Ceja, D.C., D. Ortiz-Masia, P. Salvador, L. Gisbert-Ferrandiz, C. Hernandez, M. Hausmann, et al. 2019. Succinate receptor mediates intestinal inflammation and fibrosis. Mucosal Immunology 12 (1): 178–187.
Rubic, T., G. Lametschwandtner, S. Jost, S. Hinteregger, J. Kund, N. Carballido-Perrig, et al. 2008. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nature Immunology 9 (11): 1261–1269.
Erhardt, A., and G. Tiegs. 2010. Tolerance induction in response to liver inflammation. Digestive Diseases 28 (1): 86–92.
Gatselis, N.K., K. Zachou, G.K. Koukoulis, and G.N. Dalekos. 2015. Autoimmune hepatitis, one disease with many faces: Etiopathogenetic, clinico-laboratory and histological characteristics. World Journal of Gastroenterology 21 (1): 60–83.
Sebode, M., J. Hartl, D. Vergani, A.W. Lohse, Int Autoimmune hepatitis Grp I. 2018. Autoimmune hepatitis: From current knowledge and clinical practice to future research agenda. Liver International 38 (1): 15–22.
Wang, H.-X., M. Liu, S.-Y. Weng, J.-J. Li, C. Xie, H.-L. He, et al. 2012. Immune mechanisms of Concanavalin A model of autoimmune hepatitis. World Journal of Gastroenterology 18 (2): 119–125.
Neumann, K., K. Karimi, J. Meiners, R. Voetlause, S. Steinmann, W. Dammermann, et al. 2017. A proinflammatory role of type 2 innate lymphoid cells in murine immune-mediated hepatitis. Journal of Immunology 198 (1): 128–137.
Ives, S.J., K.S. Zaleski, C. Slocum, D. Escudero, C. Sheridan, S. Legesse, et al. 2020. The effect of succinic acid on the metabolic profile in high-fat diet-induced obesity and insulin resistance. Physiological Reports 8 (21): e14630-Article No.: e.
Hakak, Y., K. Lehmann-Bruinsma, S. Phillips, T. Le, C. Liaw, D.T. Connolly, et al. 2009. The role of the GPR91 ligand succinate in hematopoiesis. Journal of Leukocyte Biology 85 (5): 837–843.
Liu, H., H. Zhang, X. Zhang, Q. Chen, and L. Xia. 2022. Role of succinic acid in the regulation of sepsis. International Immunopharmacology 110.
Iplik, E.S., T. Catmakas, and B. Cakmakoglu. 2018. A new target for the treatment of endometrium cancer by succinic acid. Cellular and Molecular Biology 64 (1): 60–63.
Chen, H., C. Jin, L. Xie, and J. Wu. 2024. Succinate as a signaling molecule in the mediation of liver diseases. Biochimica Et Biophysica Acta-Molecular Basis of Disease 1870 (2).
Li, Y.H., S.H. Woo, D.H. Choi, and E.-H. Cho. 2015. Succinate causes α-SMA production through GPR91 activation in hepatic stellate cells. Biochemical and Biophysical Research Communications 463 (4): 853–858.
Correa, P.R.A.V., E.A. Kruglov, M. Thompson, M.F. Leite, J.A. Dranoff, and M.H. Nathanson. 2007. Succinate is a paracrine signal for liver damage. Journal of Hepatology 47 (2): 262–269.
Hatano, M., S. Sasaki, S. Ohata, Y. Shiratsuchi, T. Yamazaki, K. Nagata, et al. 2008. Effects of Kupffer cell-depletion on Concanavalin A-induced hepatitis. Cellular Immunology 251 (1): 25–30.
Schümann, J., D. Wolf, A. Pahl, K. Brune, T. Papadopoulos, N. van Rooijen, et al. 2000. Importance of Kupffer cells for T-cell-dependent liver injury in mice. American Journal of Pathology 157 (5): 1671–1683.
Dong, Z., H. Wei, R. Sun, and Z. Tian. 2007. The roles of innate immune cells in liver injury and regeneration. Cellular & Molecular Immunology 4 (4): 241–252.
Trauelsen, M., T.K. Hiron, D. Lin, J.E. Petersen, B. Breton, A.S. Husted, et al. 2021. Extracellular succinate hyperpolarizes M2 macrophages through SUCNR1/GPR91-mediated Gq signaling. Cell Reports 35 (11): 1.
Jakubowski, A., M. Sternak, K. Jablonski, M. Ciszek-Lenda, J. Marcinkiewicz, and S. Chlopicki. 2016. 1-Methylnicotinamide protects against liver injury induced by concanavalin A via a prostacyclin-dependent mechanism: A possible involvement of IL-4 and TNF-α. International Immunopharmacology 31: 98–104.
Mizuhara, H., M. Uno, N. Seki, M. Yamashita, M. Yamaoka, T. Ogawa, et al. 1996. Critical involvement of interferon gamma in the pathogenesis of T-cell activation-associated hepatitis and regulatory mechanisms of interleukin-6 for the manifestations of hepatitis. Hepatology 23 (6): 1608–1615.
Ksontini, R., D.B. Colagiovanni, M.D. Josephs, C.K. Edwards, C.L. Tannahill, C.C. Solorzano, et al. 1998. Disparate roles for TNF-α and Fas ligand in concanavalin A-induced hepatitis. Journal of Immunology 160 (8): 4082–4089.
Llambi, F., and D.R. Green. 2011. Apoptosis and oncogenesis: Give and take in the BCL-2 family. Current Opinion in Genetics & Development 21 (1): 12–20.
Edlich, F. 2018. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochemical and Biophysical Research Communications 500 (1): 26–34.
Chen, H.C., M. Kanai, A. Inoue-Yamauchi, H.C. Tu, Y.F. Huang, D.C. Ren, et al. 2015. An interconnected hierarchical model of cell death regulation by the BCL-2 family. Nature Cell Biology 17 (10): 1270.
Funding
This project was supported by Fujian Provincial Natural Science Foundation (Grant No: 2021J05278), The Health Science Foundation of Fujian Youth Program (Grant No: 2021QNB017), National Natural Science Foundation of China (Grant No: 82303109), Natural Science Foundation of Fujian Province, China (Grant No: 2022J05299), and the Cross-Strait Postdoctoral Exchange Funding Program of Fujian Province, China (Grant No: 2021B002).
Author information
Authors and Affiliations
Contributions
YC, ZC, and EC contributed to the design of the study protocol. YC and DZ performed the statistical analysis. YC, TW, and MS drew the picture. YC, ZC, and EC contributed to the writing of the study protocol and made the final corrections to this manuscript. YL finally corrected the manuscript. All authors read and approved the final manuscript. YC, ZC, and EC have contributed equally to this work and share first authorship.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The animal study protocol was approved by the Ethics Committee of Xiamen University (approval no. XMULAC2023052) and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (https://www.ncbi.nlm.nih.gov/books/NBK54050/).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Messages
• Succinic acid ameliorated ConA-induced hepatitis
• Succinic acid inhibits the infiltration of liver neutrophils and the production of inflammatory factors
• Macrophages contribute to the protective role of succinic acid against ConA-induced liver injury.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, Y., Chen, Z., Chen, E. et al. Succinic Acid Ameliorates Concanavalin A-Induced Hepatitis by Altering the Inflammatory Microenvironment and Expression of BCL-2 Family Proteins. Inflammation (2024). https://doi.org/10.1007/s10753-024-02021-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10753-024-02021-6