Abstract
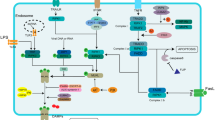
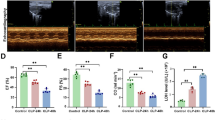
Chronic diabetes mellitus compromises the vascular system, which causes organ injury, including in the lung. Due to the strong compensatory ability of the lung, patients always exhibit subclinical symptoms. Once sepsis occurs, the degree of lung injury is more severe under hyperglycemic conditions. The α7 nicotinic acetylcholine receptor (α7nAChR) plays an important role in regulating inflammation and metabolism and can improve endothelial progenitor cell (EPC) functions. In the present study, lung injury caused by sepsis was compared between diabetic rats and normal rats. We also examined whether α7nAChR activation combined with EPC transplantation could ameliorate lung injury in diabetic sepsis rats. A type 2 diabetic model was induced in rats via a high-fat diet and streptozotocin. Then, a rat model of septic lung injury was established by intraperitoneal injection combined with endotracheal instillation of LPS. The oxygenation indices, wet-to-dry ratios, and histopathological scores of the lungs were tested after PNU282987 treatment and EPC transplantation. IL-6, IL-8, TNF-α, and IL-10 levels were measured. Caspase-3, Bax, Bcl-2, and phosphorylated NF-κB (p-NF-κB) levels were determined by blotting. Sepsis causes obvious lung injury, which is exacerbated by diabetic conditions. α7nAChR activation and endothelial progenitor cell transplantation reduced lung injury in diabetic sepsis rats, alleviating inflammation and decreasing apoptosis. This treatment was more effective when PNU282987 and endothelial progenitor cells were administered together. p-NF-κB levels decreased following treatment with PNU282987 and EPCs. In conclusion, α7nAChR activation combined with EPC transplantation can alleviate lung injury in diabetic sepsis rats through the NF-κB signaling pathway.






Similar content being viewed by others
DATA AVAILABILITY
All data generated or analysed during this study are available from the corresponding author on reasonable request.
References
Tomic, D., J.E. Shaw, and D.J. Magliano. 2022. The burden and risks of emerging complications of diabetes mellitus. Nature Reviews Endocrinology 18 (9): 525–539. https://doi.org/10.1038/s41574-022-00690-7.
Mauricio, D., N. Alonso, and M. Gratacos. 2020. Chronic diabetes complications: The need to move beyond classical concepts. Trends in Endocrinology and Metabolism 31 (4): 287–295. https://doi.org/10.1016/j.tem.2020.01.007.
Zheng, H., J. Wu, Z. Jin, et al. 2017. Potential biochemical mechanisms of lung injury in diabetes. Aging and Disease 8 (1): 7–16. https://doi.org/10.14336/AD.2016.0627.
Ugan, R.A., E. Cadirci, Z. Halici, et al. 2018. The role of urotensin-II and its receptors in sepsis-induced lung injury under diabetic conditions. European Journal of Pharmacology 818: 457–469. https://doi.org/10.1016/j.ejphar.2017.11.011.
Visca, D., P. Pignatti, A. Spanevello, et al. 2018. Relationship between diabetes and respiratory diseases-clinical and therapeutic aspects. Pharmacological Research 137: 230–235. https://doi.org/10.1016/j.phrs.2018.10.008.
Xie, H., N. Yepuri, Q. Meng, et al. 2020. Therapeutic potential of alpha7 nicotinic acetylcholine receptor agonists to combat obesity, diabetes, and inflammation. Reviews in Endocrine & Metabolic Disorders 21 (4): 431–447. https://doi.org/10.1007/s11154-020-09584-3.
Shao, Z., Q. Li, S. Wang, et al. 2019. Protective effects of PNU282987 on sepsisinduced acute lung injury in mice. Molecular Medicine Reports 19 (5): 3791–3798. https://doi.org/10.3892/mmr.2019.10016.
Bouzat, C., M. Lasala, B.E. Nielsen, et al. 2018. Molecular function of alpha7 nicotinic receptors as drug targets. Journal of Physiology 596 (10): 1847–1861. https://doi.org/10.1113/JP275101.
Pavlov, V.A., and K.J. Tracey. 2005. The cholinergic anti-inflammatory pathway. Brain, Behavior, and Immunity 19 (6): 493–499. https://doi.org/10.1016/j.bbi.2005.03.015.
Gausseres, B., J. Liu, E. Foppen, et al. 2020. The constitutive lack of alpha7 nicotinic receptor leads to metabolic disorders in mouse. Biomolecules 10 (7). PMID 32708537. https://doi.org/10.3390/biom10071057.
Youssef, M.E., H.M. Abdelrazek, and Y.M. Moustafa. 2021. Cardioprotective role of GTS-21 by attenuating the TLR4/NF-kappaB pathway in streptozotocin-induced diabetic cardiomyopathy in rats. Naunyn-Schmiedeberg’s Archives of Pharmacology 394 (1): 11–31. https://doi.org/10.1007/s00210-020-01957-4.
Marrero, M.B., R. Lucas, C. Salet, et al. 2010. An alpha7 nicotinic acetylcholine receptor-selective agonist reduces weight gain and metabolic changes in a mouse model of diabetes. Journal of Pharmacology and Experimental Therapeutics 332 (1): 173–180. https://doi.org/10.1124/jpet.109.154633.
Gupta, D., A.A. Lacayo, S.M. Greene, et al. 2018. β-Cell mass restoration by alpha7 nicotinic acetylcholine receptor activation. Journal of Biological Chemistry 293 (52): 20295–20306. https://doi.org/10.1074/jbc.RA118.004617.
Vincent, J.L., C. Ince, and P. Pickkers. 2021. Endothelial dysfunction: A therapeutic target in bacterial sepsis? Expert Opinion on Therapeutic Targets 25 (9): 733–748. https://doi.org/10.1080/14728222.2021.1988928.
Cribbs, S.K., M.A. Matthay, and G.S. Martin. 2010. Stem cells in sepsis and acute lung injury. Critical Care Medicine 38 (12): 2379–2385. https://doi.org/10.1097/CCM.0b013e3181f96f5f.
Liew, A., J.H. McDermott, F. Barry, et al. 2008. Endothelial progenitor cells for the treatment of diabetic vasculopathy: Panacea or Pandora’s box? Diabetes, Obesity & Metabolism 10 (5): 353–366. https://doi.org/10.1111/j.1463-1326.2007.00754.x.
Leone, A.M., M. Valgimigli, M.B. Giannico, et al. 2009. From bone marrow to the arterial wall: The ongoing tale of endothelial progenitor cells. European Heart Journal 30 (8): 890–899. https://doi.org/10.1093/eurheartj/ehp078.
Sun, R., J. Huang, and B. Sun. 2020. Mobilization of endothelial progenitor cells in sepsis. Inflammation Research 69 (1): 1–9. https://doi.org/10.1007/s00011-019-01299-9.
Krenning, G., M.J. van Luyn, and M.C. Harmsen. 2009. Endothelial progenitor cell-based neovascularization: Implications for therapy. Trends in Molecular Medicine 15 (4): 180–189. https://doi.org/10.1016/j.molmed.2009.02.001.
Ma, W., W. Zhang, B. Cui, et al. 2021. Functional delivery of lncRNA TUG1 by endothelial progenitor cells derived extracellular vesicles confers anti-inflammatory macrophage polarization in sepsis via impairing miR-9-5p-targeted SIRT1 inhibition. Cell Death & Disease 12 (11): 1056. https://doi.org/10.1038/s41419-021-04117-5.
Zhou, Y., P. Li, A.J. Goodwin, et al. 2019. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Critical Care 23 (1): 44. https://doi.org/10.1186/s13054-019-2339-3.
Zhou, Y., P. Li, A.J. Goodwin, et al. 2018. Exosomes from endothelial progenitor cells improve the outcome of a murine model of sepsis. Molecular Therapy 26 (5): 1375–1384. https://doi.org/10.1016/j.ymthe.2018.02.020.
Li, X., C. Chen, L. Wei, et al. 2016. Exosomes derived from endothelial progenitor cells attenuate vascular repair and accelerate reendothelialization by enhancing endothelial function. Cytotherapy 18 (2): 253–262. https://doi.org/10.1016/j.jcyt.2015.11.009.
Bai, S., Q. Yin, T. Dong, et al. 2020. Endothelial progenitor cell-derived exosomes ameliorate endothelial dysfunction in a mouse model of diabetes. Biomedicine & Pharmacotherapy 131: 110756. https://doi.org/10.1016/j.biopha.2020.110756.
Rafat, N., C. Hanusch, P.T. Brinkkoetter, et al. 2007. Increased circulating endothelial progenitor cells in septic patients: Correlation with survival. Critical Care Medicine 35 (7): 1677–1684. https://doi.org/10.1097/01.CCM.0000269034.86817.59.
Yiu, K.H., and H.F. Tse. 2014. Specific role of impaired glucose metabolism and diabetes mellitus in endothelial progenitor cell characteristics and function. Arteriosclerosis, Thrombosis, and Vascular Biology 34 (6): 1136–1143. https://doi.org/10.1161/ATVBAHA.114.302192.
Desouza, C.V., F.G. Hamel, K. Bidasee, et al. 2011. Role of inflammation and insulin resistance in endothelial progenitor cell dysfunction. Diabetes 60 (4): 1286–1294. https://doi.org/10.2337/db10-0875.
Luo, T.H., Y. Wang, Z.M. Lu, et al. 2009. The change and effect of endothelial progenitor cells in pig with multiple organ dysfunction syndromes. Critical Care 13 (4): R118. https://doi.org/10.1186/cc7968.
Zhang, X., G. Mao, Z. Zhang, et al. 2020. Activating alpha7nAChRs enhances endothelial progenitor cell function partially through the JAK2/STAT3 signaling pathway. Microvascular Research 129: 103975. https://doi.org/10.1016/j.mvr.2020.103975.
Chen, X., J. Chen, Y. Song, et al. 2020. Vagal alpha7nAChR signaling regulates alpha7nAChR(+)Sca1(+) cells during lung injury repair. Stem Cell Research & Therapy 11 (1): 375. https://doi.org/10.1186/s13287-020-01892-4.
Jiang, T., Y. Liu, Q. Meng, et al. 2019. Hydrogen sulfide attenuates lung ischemia-reperfusion injury through SIRT3-dependent regulation of mitochondrial function in type 2 diabetic rats. Surgery 165 (5): 1014–1026. https://doi.org/10.1016/j.surg.2018.12.018.
Ji, Y., G. Zhang, H. Zhu, et al. 2018. Indinavir plus methylprednisolone ameliorates experimental acute lung injury in vitro and in vivo. Shock 49 (2): 196–204. https://doi.org/10.1097/SHK.0000000000000911.
Jin, Z., M.Y. Li, L. Tang, et al. 2022. Protective effect of ulinastatin on acute lung injury in diabetic sepsis rats. International Immunopharmacology 108: 108908. https://doi.org/10.1016/j.intimp.2022.108908.
Li, S., D. Qi, J.N. Li, et al. 2021. Vagus nerve stimulation enhances the cholinergic anti-inflammatory pathway to reduce lung injury in acute respiratory distress syndrome via STAT3. Cell Death Discovery 7 (1): 63. https://doi.org/10.1038/s41420-021-00431-1.
Pinheiro, N.M., R. Banzato, I. Tiberio, et al. 2021. Acute lung injury in cholinergic-deficient mice supports anti-inflammatory role of alpha7 nicotinic acetylcholine receptor. International Journal of Molecular Sciences 22 (14). PMID 34299169. https://doi.org/10.3390/ijms22147552.
Meng, Q., O.G. Chepurny, C.A. Leech, et al. 2022. The alpha-7 nicotinic acetylcholine receptor agonist GTS-21 engages the glucagon-like peptide-1 incretin hormone axis to lower levels of blood glucose in db/db mice. Diabetes, Obesity & Metabolism 24 (7): 1255–1266. https://doi.org/10.1111/dom.14693.
Zhang, R.H., J.B. Zhou, Y.H. Cai, et al. 2020. Non-linear association between diabetes mellitus and pulmonary function: A population-based study. Respiratory Research 21 (1): 292. https://doi.org/10.1186/s12931-020-01538-2.
Filippini, P., A. Cesario, M. Fini, et al. 2012. The yin and yang of non-neuronal alpha7-nicotinic receptors in inflammation and autoimmunity. Current Drug Targets 13 (5): 644–655. https://doi.org/10.2174/138945012800399008.
Schuller, H.M. 2012. Regulatory role of the alpha7nAChR in cancer. Current Drug Targets 13 (5): 680–687. https://doi.org/10.2174/138945012800398883.
Pillai, S., and S. Chellappan. 2012. alpha7 nicotinic acetylcholine receptor subunit in angiogenesis and epithelial to mesenchymal transition. Current Drug Targets 13 (5): 671–679. https://doi.org/10.2174/138945012800398847.
Gundavarapu, S., J.A. Wilder, N.C. Mishra, et al. 2012. Role of nicotinic receptors and acetylcholine in mucous cell metaplasia, hyperplasia, and airway mucus formation in vitro and in vivo. J Allergy Clin Immunol 130 (3): 770–780.e11. https://doi.org/10.1016/j.jaci.2012.04.002.
Douaoui, S., R. Djidjik, M. Boubakeur, et al. 2020. GTS-21, an alpha7nAChR agonist, suppressed the production of key inflammatory mediators by PBMCs that are elevated in COPD patients and associated with impaired lung function. Immunobiology 225 (3): 151950. https://doi.org/10.1016/j.imbio.2020.151950.
Su, X., M.A. Matthay, and A.B. Malik. 2010. Requisite role of the cholinergic alpha7 nicotinic acetylcholine receptor pathway in suppressing Gram-negative sepsis-induced acute lung inflammatory injury. The Journal of Immunology 184 (1): 401–410. https://doi.org/10.4049/jimmunol.0901808.
Lafargue, M., L. Xu, M. Carles, et al. 2012. Stroke-induced activation of the alpha7 nicotinic receptor increases Pseudomonas aeruginosa lung injury. The FASEB Journal 26 (7): 2919–2929. https://doi.org/10.1096/fj.11-197384.
Engel, O., L. Akyuz, A.C. da Costa Goncalves, et al. 2015. Cholinergic pathway suppresses pulmonary innate immunity facilitating pneumonia after stroke. Stroke 46 (11): 3232–3240. https://doi.org/10.1161/STROKEAHA.115.008989.
Giebelen, I.A., M. Leendertse, S. Florquin, et al. 2009. Stimulation of acetylcholine receptors impairs host defence during pneumococcal pneumonia. European Respiratory Journal 33 (2): 375–381. https://doi.org/10.1183/09031936.00103408.
Hajiasgharzadeh, K., S. Sadigh-Eteghad, B. Mansoori, et al. 2019. Alpha7 nicotinic acetylcholine receptors in lung inflammation and carcinogenesis: Friends or foes? Journal of Cellular Physiology 234 (9): 14666–14679. https://doi.org/10.1002/jcp.28220.
Mokra, D., and P. Kosutova. 2015. Biomarkers in acute lung injury. Respiratory Physiology & Neurobiology 209: 52–58. https://doi.org/10.1016/j.resp.2014.10.006.
Kawasaki, T., T. Nishiwaki, A. Sekine, et al. 2015. Vascular repair by tissue-resident endothelial progenitor cells in endotoxin-induced lung injury. American Journal of Respiratory Cell and Molecular Biology 53 (4): 500–512. https://doi.org/10.1165/rcmb.2014-0185OC.
Zampetaki, A., J.P. Kirton, and Q. Xu. 2008. Vascular repair by endothelial progenitor cells. Cardiovascular Research 78 (3): 413–421. https://doi.org/10.1093/cvr/cvn081.
Fadini, G.P., and A. Avogaro. 2010. Potential manipulation of endothelial progenitor cells in diabetes and its complications. Diabetes, Obesity & Metabolism 12 (7): 570–583. https://doi.org/10.1111/j.1463-1326.2010.01210.x.
Xing, Z., C. Zhao, H. Liu, et al. 2020. Endothelial progenitor cell-derived extracellular vesicles: A novel candidate for regenerative medicine and disease treatment. Advance Healthcare Materials 9 (12): e2000255. https://doi.org/10.1002/adhm.202000255.
Zingarelli, B. 2005. Nuclear factor-kappaB. Critical Care Medicine 33 (12 Suppl): S414–S416. https://doi.org/10.1097/01.ccm.0000186079.88909.94.
Donath, M.Y. 2013. Targeting inflammation in the treatment of type 2 diabetes. Diabetes, Obesity & Metabolism 15 (Suppl 3): 193–196. https://doi.org/10.1111/dom.12172.
Chian, C.F., C.H. Chiang, C.H. Chuang, et al. 2014. Inhibitor of nuclear factor-kappaB, SN50, attenuates lipopolysaccharide-induced lung injury in an isolated and perfused rat lung model. Translational Research 163 (3): 211–220. https://doi.org/10.1016/j.trsl.2013.10.002.
Kassan, M., S.K. Choi, M. Galan, et al. 2013. Enhanced NF-kappaB activity impairs vascular function through PARP-1-, SP-1-, and COX-2-dependent mechanisms in type 2 diabetes. Diabetes 62 (6): 2078–2087. https://doi.org/10.2337/db12-1374.
Meyerovich, K., M. Fukaya, L.F. Terra, et al. 2016. The non-canonical NF-kappaB pathway is induced by cytokines in pancreatic beta cells and contributes to cell death and proinflammatory responses in vitro. Diabetologia 59 (3): 512–521. https://doi.org/10.1007/s00125-015-3817-z.
Funding
This study was supported by a grant from the Natural Science Foundation of Heilongjiang Province (grant no. LH2021H026).
Author information
Authors and Affiliations
Contributions
Conception and design of the study: Wengang Ding, Xiaoyun Zhang, Haixu Wang, Xuemin Cai. Acquisition of the data: Xiaoyun Zhang, Haixu Wang and Xuemin Cai. Analysis and/or interpretation of the data: Wengang Ding, Xiaoyun Zhang, Haixu Wang, Xuemin Cai, Enran Liu, Zhiyuan Li, Tao Jiang. Drafting the manuscript: Xiaoyun Zhang. Revising the manuscript critically for important intellectual content: Wengang Ding, Xiaoyun Zhang, Haixu Wang, Xuemin Cai, Enran Liu, Zhiyuan Li, Tao Jiang, Dongmei Li. Approval of the version of the manuscript to be published: Wengang Ding, Xiaoyun Zhang, Haixu Wang, Xuemin Cai, Enran Liu, Zhiyuan Li, Tao Jiang, Dongmei Li. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All animal protocols were approved by the Animal Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (approval no. SYDW2021-009).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Wang, H., Cai, X. et al. α7nAChR Activation Combined with Endothelial Progenitor Cell Transplantation Attenuates Lung Injury in Diabetic Rats with Sepsis through the NF-κB Pathway. Inflammation (2024). https://doi.org/10.1007/s10753-024-01980-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10753-024-01980-0