Abstract
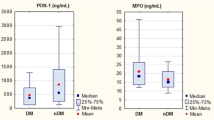
Inflammation and oxidative stress play a significant role in the pathogenesis of acute myeloid leukemia. While myeloperoxidase carries pro-oxidant effects, HDL-cholesterol and paraoxonase have antioxidant properties. Therefore, we evaluated serum paraoxonase, myeloperoxidase, and HDL-cholesterol levels in cases with acute myeloid leukemia. Myeloperoxidase, paraoxonase, and HDL-cholesterol levels in 40 acute myeloid leukemia patients and 18 healthy individuals were determined. The relationship between these parameters and other prognostic factors, as well as their association with response to chemotherapy, was investigated. Myeloperoxidase levels were higher, while paraoxonase and HDL-cholesterol levels were lower in acute myeloid leukemia cases compared to the control group (p < 0.001, p < 0.001, p = 0.006, respectively). The myeloperoxidase level was significantly negatively correlated with paraoxonase and HDL-c levels (r = − 0.64, p < 0.001; r = − 0.27, p = 0.02, respectively). Paraoxonase level was positively correlated with HDL level (r = 0.34, p = 0.04). Lactate dehydrogenase level was negatively correlated with HDL-c and paraoxonase levels and positively correlated with myeloperoxidase level (r = − 0.37, p = 0.019; r = − 0.35, p = 0.04; r = 0.45, p = 0.03, respectively). Following complete remission induction treatment, cases with complete remission had lower myeloperoxidase levels and higher HDL-cholesterol and paraoxonase levels compared to other cases (p = 0.03, p = 0.01, p = 0.04, respectively). Myeloperoxidase levels are higher, while paraoxonase and HDL-cholesterol levels are lower in acute myeloid leukemia cases. The obtained findings emphasize the potential importance of inflammation and oxidative stress in the pathogenesis of acute myeloid leukemia. These parameters can be used as biomarkers for prognosis prediction and prediction of response to chemotherapy.
Similar content being viewed by others
Data Availability
The datasets generated or analyzed during the current study are available on request from the corresponding authors.
References
Prieto-Bermejo, R., M. Romo-González, A. Pérez-Fernández, C. Ijurko, and A. Hernández-Hernández. 2018. Reactive oxygen species in haematopoiesis: Leukaemic cells take a walk on the wild side. Journal of Experimental & Clinical Cancer Research 37 (1): 125–142.
Trachootham, D., J. Alexandre, and P. Huang. 2009. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nature Reviews. Drug Discovery 8 (7): 579–591.
Singh, N., D. Baby, J.P. Rajguru, P.B. Patil, S.S. Thakkannavar, V.B. Pujari VB. 2019. Inflammation and cancer. Annals of African Medicine 18 (3): 121–126.
Rybicka-Ramos, M., M. Markiewicz, A. Suszka-Switek, R. Wiaderkiewicz, S. Mizia, M. Dzierzak-Mietla, and K. Bialas. 2021. Proinflammatory cytokines as potential risk factors of acute graft-versus-host disease and infectious complications after allogeneic hematopoietic stem cell transplantation. Am J Blood Res. 11 (2): 149–156.
Blatter, MC., R.W. James, S. Messmer, F. Barja, D. Pometta. 1993. Identification of a distinct human high-density lipoprotein subspecies defined by a lipoprotein-associated protein, K-45. Identity of K-45 with paraoxonase. European Journal of Biochemistry 211 (3): 871–879.
Deakin, S., I. Leviev, M. Gomaraschi, L. Calabresi, G. Franceschini, and R.W. James. 2002. Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high affinity, saturable, desorption mechanism. Journal of Biological Chemistry 277 (6): 4301–4308.
Wang, Y., J. Zhang, Y. Hu, L. Yang, J. Xue, and Y. Ding. 2019. Relationship between paraoxonase-1 activity and lipid profiles in Chinese individuals with or without coronary artery disease. Lipids in Health and Disease. 18 (1): 45.
Mackness, MI., B. Mackness, P.N. Durrington, P.W. Connelly, R.A. Hegele. 1996. Paraoxonase: Biochemistry, genetics, and relationship to plasma lipoproteins. Current Opinion in Lipidology 7: 69–76.
Nevin, DN., A. Zambon, C.E. Furlong, R.J. Richter, R. Humbert, J.E. Hokanson, J.D. Brunzell. 1996. Paraoxonase genotypes, lipoprotein lipase activity, and HDL. Arteriosclerosis, Thrombosis, and Vascular Biology 16: 1243–1249.
Costa, LG., A. Vitalone, T.B. Cole, C.E. Furlong. 2005. Modulation of paraoxonase (PON1) activity. Biochemical Pharmacology 69: 541–550.
Baum, L., H.K. Ng, K.S. Woo, B. Tomlinson, T.H. Rainer, X. Chen, W.S. Cheung, D.K. Chan, G.N. Thomas, C.S. Tong, and K.S. Wong. 2006. Paraoxonase 1 gene Q192R polymorphism affects stroke and myocardial infarction risk. Clinical Biochemistry 39: 191–195.
Gokdemir, MT., A.Z. Karakilcik, G.S. Gokdemir. 2017. Prognostic importance of paraoxonase, arylesterase and mean platelet volume efficiency in acute ischaemic stroke. Journal of Pakistan Medical Association 67: 1679–1683.
Utanğaç, MM., E. Yeni, M. Savaş, A. Altunkol, H. Çiftçi, K. Gümüş, M. Demir. 2017. Paraoxonase and arylesterase activity in bladder cancer. Turkish Journal of Urology 43: 147–51.
Kul, A., H. Uzkeser, and N. Ozturk. 2017. Paraoxonase and arylesterase levels in Behcet’s disease and their relations with the disease activity. Biochemical Genetics 55: 335–344.
Khan, AA., M.A. Alsahli, A.H. Rahmani. 2018. Myeloperoxidase as an active disease biomarker: Recent biochemical and pathological perspectives. Medical Sciences (Basel) 6 (2): 33.
Anatoliotakis, N., S. Deftereos, G. Bouras, G. Giannopoulos, D. Tsounis, C. Angelidis, A. Kaoukis, and C. Stefanadis. 2013. Myeloperoxidase: Expressing inflammation and oxidative stress in cardiovascular disease. Current Topics in Medicinal Chemistry 13 (2): 115–138.
Ajila, V., V. Ravi, S. Kumari, S. Babu, S. Hegde, and A. Madiyal. 2015. Serum and salivary myeloperoxidase in oral squamous cell carcinoma: A preliminary study. Clin Cancer Investig J. 4 (3): 344–348.
Lai, WM., C.C. Chen, J.H. Lee, C.J. Chen, J.S. Wang, Y.Y. Hou, H.H. Liou, H.C. Chen, T.Y. Fu, Y.C. Lee, L.P. Ger. 2013. Second primary tumors and myeloperoxidase expression in buccal mucosal squamous cell carcinoma. Oral Surgery, Oral Medicine, Oral Pathology, and Oral Radiology 116 (4): 464–473.
Qin, X., Y. Deng, Z.Y. Zeng, Q.L. Peng, X.L. Huang, C.J. Mo, S. Li, and J.M. Zhao. 2013. Myeloperoxidase polymorphism, menopausal status, and breast cancer risk: An update meta-analysis. PLoS ONE 8 (8): e72583.
Arslan, S., H. Pinarbasi, and Y. Silig. 2011. Myeloperoxidase G-463A polymorphism and risk of lung and prostate cancer in a Turkish population. Molecular Medicine Reports 4 (1): 87–92.
Jilani, I., T. Vincenti, H. Faraji, F.J. Giles, E. Estey, H.M. Kantarjian, and M. Albitar. 2004. Clinical relevance of circulating myeloperoxidase (MPO) in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). Blood 104 (11): 1073.
Gonçalves, RP., D.G. Rodrigues, R.C. Maranhão. 2005. Uptake of high density lipoprotein (HDL) cholesteryl esters by human acute leukemia cells. Leukemia Research 29 (8): 955–959.
Kuliszkiewicz-Janus, M., R. Małecki, and A.S. Mohamed. 2008. Lipid changes occuring in the course of hematological cancers. Cellular & Molecular Biology Letters 13: 465–474.
Rye, KA., C.A. Bursill, G. Lambert, F. Tabet, P.J. Barter. 2009. The metabolism and anti-atherogenic properties of HDL. Journal of Lipid Research 50: 195–200.
Undurti, A., Y. Huang, J.A. Lupica, J.D. Smith, J.A. DiDonato, and S.L. Hazen. 2009. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. Journal of Biological Chemistry 284 (45): 30825–30835.
Eckerson, EW., J. Romson, C. Wyte, B.N. La Du. 1983. The human serum paraoxonase polymorphism: Identification of phenotypes by their response to salts. American Journal of Human Genetics 35: 214–227.
Bradly, PP., D.A. Priebat, R.D. Christensen, G. Rathstein. 1982. Measurement of cutaneous inflammations estimation of neutrophil content with an enzyme marker. Journal of Investigative Dermatology 78 (3): 206–209.
Akçay, MN., I. Yilmaz, M.F. Polat, G. Akçay. 2003. Serum paraoxonase levels in gastric cancer. Hepatogastroenterology 50: cclxxiii-cclxxv.
Elkiran, ET., N. Mar, B. Aygen, F. Gursu, A. Karaoglu, S. Koca. 2007. Serum paraoxonase and arylesterase activities in patients with lung cancer in a Turkish population. BMC Cancer 15: 48.
Samra, ZQ., S. Pervaiz, S. Shaheen, N. Dar, M.A. Athar. 2011. Determination of oxygen derived free radicals producer (xanthine oxidase) and scavenger (paraoxonase1) enzymes and lipid parameters in different cancer patients. Clinical Laboratory 57 (9–10): 741–747.
Çebi, A., E. Akgun, R. Esen, H. Demir, and A. Çifci. 2015. The activities of serum paraoxonase and arylesterase and lipid profile in acute myeloid leukemia: Preliminary results. European Review for Medical and Pharmacological Sciences 19 (23): 4590–4594.
Bacchetti, T., G. Ferretti, and A. Sahebkar. 2019. The role of paraoxonase in cancer. Seminars in Cancer Biology 56: 72–86.
Morsia, E., S. Rupoli, E. Molinelli, D. Sartini, A.M. Ofdani, M. Emanuelli, E. Salvolini, A. Olivieri, and G. Goteri. 2021. Expression of paraoxonase-2 in different stages of cutaneous T cell lymphoma: Is it a potential biomarker for aggressiveness? Blood 138: 4496–4497.
Ellidag, HY., E. Eren, O. Aydin, M. Yıldırım, C. Sezer, N. Yilmaz. 2014. Multiple myeloma: Relationship to antioxidant esterases. Medical Principles and Practice 23 (1): 18–23.
Musolino, C., L. Calabrò, G. Bellomo, M. Cincotta, V. Di Giacomo, C. Pezzano, B. Loteta, V. Rizzo, S. Guglielmo, and A. Alonci. 2002. Lipid profile in hematologic neoplasms. Rec Prog Med. 93: 298–301.
Lim, U., T. Gayles, H.A. Katki, R. Stolzenberg-Solomon, S.J. Weinstein, P. Pietinen, P.R. Taylor, J. Virtamo, and D. Albanes. 2007. Serum high-density lipoprotein cholesterol and risk of non-Hodgkin lymphoma. Cancer Research 67 (11): 5569–5574.
Fiorenza, AM., A. Branchi, D. Sommariva. 2000. Serum lipoprotein profile in patients with cancer. A comparison with non-cancer subjects. International Journal of Clinical and Laboratory Research 30 (3): 141–145.
Baroni, S., D. Scribano, C. Zuppi, L. Pagano, G. Leone, and B. Giardina. 1996. Prognostic relevance of lipoprotein cholesterol levels in acute lymphocytic and nonlymphocytic leukemia. Acta Haematologica 96 (1): 24–28.
Wang, L., P.D. Chi, H. Chen, J. Xiang, Z.J. Xia, and Y.J. Zhang. 2014. Low level of high-density lipoprotein cholesterol correlates with poor prognosis in extranodal natural killer/T cell lymphoma. Tumour Biology 35 (3): 2141–2149.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection was performed Gülden Sincan. Data analysis was performed Suat Sincan. Material preparation was made Suat Sincan, Gülden Sincan, Seda Taşkın, Ahmet Kızıltunç. The first draft of the manuscript was written by Suat Sincan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University Atatürk (Date: 29.12.2022, No: B.30.2.ATA.0.01.00/69).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sincan, S., Sincan, G., Aşkın, S. et al. Evaluation of Serum Paraoxonase, Myeloperoxidase, and HDL-Cholesterol Levels in Acute Myeloid Leukemia. Inflammation 46, 2470–2476 (2023). https://doi.org/10.1007/s10753-023-01924-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01924-0