Abstract—
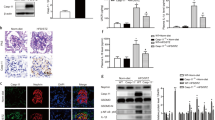
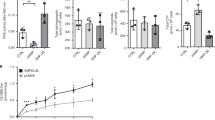
The activation of nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3 (NLRP3) inflammasome has been reported to importantly contribute to glomerular inflammation and injury under different pathological conditions such as obesity. However, the mechanism mediating NLRP3 inflammasome activation in podocytes and subsequent glomerular injury remains poorly understood. Given that the ceramide signaling pathway has been reported to be implicated in obesity-related glomerulopathy (ORG), the present study was designed to test whether the ceramide-producing enzyme, acid sphingomyelinase (ASM), determines NLRP3 inflammasome activation and inflammatory exosome release in podocytes leading to glomerular inflammation and injury during ORG. In Smpd1trg/Podocre mice, podocyte-specific overexpression of Smpd1 gene which encodes ASM significantly exaggerated high-fat diet (HFD)-induced NLRP3 inflammasome activation in podocytes and immune cell infiltration in glomeruli compared to WT/WT mice. Smpd1 gene deletion, however, blocked these pathological changes induced by HFD in Smpd1−/− mice. Accompanied with NLRP3 inflammasome activation and glomerular inflammation, urinary excretion of exosomes containing podocyte marker and NLRP3 inflammasome products (IL-1β and IL-18) in Smpd1trg/Podocre mice on the HFD was much higher than that in WT/WT mice. In contrast, Smpd1−/− mice on the HDF had significantly lower urinary exosome excretion than WT/WT mice. Correspondingly, HFD-induced podocyte injury, glomerular sclerosis, and proteinuria were more severe in Smpd1trg/Podocre mice, but milder in Smpd1−/− mice compared to WT/WT mice. Using podocytes isolated from these mice, we demonstrated that visfatin, a prototype pro-inflammatory adipokine, induced NLRP3 inflammasome activation and enrichment of multivesicular bodies (MVBs) containing IL-1β in podocytes, which was much stronger in podocytes from Smpd1trg/Podocre mice, but weaker in those from Smpd1−/− mice than WT/WT podocytes. By quantitative analysis of exosomes, it was found that upon visfatin stimulation, podocytes from Smpd1trg/Podocre mice released much more exosomes containing NLRP3 inflammasome products, but podocytes from Smpd1−/− mice released much less exosomes compared to WT/WT podocytes. Super-resolution microscopy demonstrated that visfatin inhibited lysosome-MVB interaction in podocytes, indicating impaired MVB degradation by lysosome. The inhibition of lysosome-MVB interaction by visfatin was amplified by Smpd1 gene overexpression but attenuated by Smpd1 gene deletion. Taken together, our results suggest that ASM in podocytes is a crucial regulator of NLRP3 inflammasome activation and inflammatory exosome release that instigate glomerular inflammation and injury during obesity.








Similar content being viewed by others
Availability of Data and Materials
The data used to support the findings of this study are available from the corresponding author upon request.
References
Martinon, F., A. Mayor, and J. Tschopp. 2009. The inflammasomes: Guardians of the body. Annual Review of Immunology 27: 229–265.
Abais, J.M., C. Zhang, M. Xia, Q. Liu, T.W. Gehr, K.M. Boini, et al. 2013. NADPH oxidase-mediated triggering of inflammasome activation in mouse podocytes and glomeruli during hyperhomocysteinemia. Antioxidants & redox signaling 18: 1537–1548.
Boini, K.M., M. Xia, S. Koka, T.W. Gehr, and P.L. Li. 2016. Instigation of NLRP3 inflammasome activation and glomerular injury in mice on the high fat diet: Role of acid sphingomyelinase gene. Oncotarget 7: 19031–19044.
Cruz, C.M., A. Rinna, H.J. Forman, A.L. Ventura, P.M. Persechini, and D.M. Ojcius. 2007. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. Journal of Biological Chemistry 282: 2871–2879.
Halle, A., V. Hornung, G.C. Petzold, C.R. Stewart, B.G. Monks, T. Reinheckel, et al. 2008. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nature Immunology 9: 857–865.
Nour, A.M., Y.G. Yeung, L. Santambrogio, E.D. Boyden, E.R. Stanley, and J. Brojatsch. 2009. Anthrax lethal toxin triggers the formation of a membrane-associated inflammasome complex in murine macrophages. Infection and Immunity 77: 1262–1271.
Chen, G.Y., and G. Nunez. 2010. Sterile inflammation: Sensing and reacting to damage. Nature Reviews Immunology 10: 826–837.
Lamkanfi, M. 2011. Emerging inflammasome effector mechanisms. Nature Reviews Immunology 11: 213–220.
Martinon, F., K. Burns, and J. Tschopp. 2002. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular Cell 10: 417–426.
Srinivasula, S.M., J.L. Poyet, M. Razmara, P. Datta, Z. Zhang, and E.S. Alnemri. 2002. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. Journal of Biological Chemistry 277: 21119–21122.
Boini, K.M., M. Xia, J.M. Abais, G. Li, A.L. Pitzer, T.W. Gehr, et al. 2014. Activation of inflammasomes in podocyte injury of mice on the high fat diet: Effects of ASC gene deletion and silencing. Biochimica et Biophysica Acta 1843: 836–845.
Griffiths, G., and K. Simons. 1986. The trans Golgi network: Sorting at the exit site of the Golgi complex. Science 234: 438–443.
Gu, F., C.M. Crump, and G. Thomas. 2001. Trans-Golgi network sorting. Cellular and molecular life sciences : CMLS 58: 1067–1084.
Colombo, M., G. Raposo, and C. Thery. 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell and Developmental Biology 30: 255–289.
Schorey, J.S., and C.V. Harding. 2016. Extracellular vesicles and infectious diseases: New complexity to an old story. The Journal of Clinical Investigation 126: 1181–1189.
Li, G., J. Kidd, and P.L. Li. 2020. Podocyte lysosome dysfunction in chronic glomerular diseases. International journal of molecular sciences 21.
Takahashi, A., R. Okada, K. Nagao, Y. Kawamata, A. Hanyu, S. Yoshimoto, et al. 2017. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nature Communications 8: 15287.
Eitan, E., C. Suire, S. Zhang, and M.P. Mattson. 2016. Impact of lysosome status on extracellular vesicle content and release. Ageing research reviews 32: 65–74.
van Balkom, B.W., T. Pisitkun, M.C. Verhaar, and M.A. Knepper. 2011. Exosomes and the kidney: Prospects for diagnosis and therapy of renal diseases. Kidney International 80: 1138–1145.
Zhou, H., H. Kajiyama, T. Tsuji, X. Hu, A. Leelahavanichkul, S. Vento, et al. 2013. Urinary exosomal Wilms’ tumor-1 as a potential biomarker for podocyte injury. American Journal of Physiology. Renal Physiology 305: F553–F559.
Erdbrugger, U., and T.H. Le. 2016. Extracellular vesicles in renal diseases: More than novel biomarkers? Journal of the American Society of Nephrology 27: 12–26.
Hara, M., T. Yanagihara, I. Kihara, K. Higashi, K. Fujimoto, and T. Kajita. 2005. Apical cell membranes are shed into urine from injured podocytes: A novel phenomenon of podocyte injury. Journal of the American Society of Nephrology: JASN 16: 408–416.
Lee, H., K.H. Han, S.E. Lee, S.H. Kim, H.G. Kang, and H.I. Cheong. 2012. Urinary exosomal WT1 in childhood nephrotic syndrome. Pediatric Nephrology(Berlin, Germany) 27: 317–320.
Lytvyn, Y., F. Xiao, C.R. Kennedy, B.A. Perkins, H.N. Reich, J.W. Scholey, et al. 2017. Assessment of urinary microparticles in normotensive patients with type 1 diabetes. Diabetologia 60: 581–584.
Stahl, A.L., K. Johansson, M. Mossberg, R. Kahn, and D. Karpman. 2019. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatric nephrology 34: 11–30.
Tkaczyk, M., and Z. Baj. 2002. Surface markers of platelet function in idiopathic nephrotic syndrome in children. Pediatric nephrology 17: 673–677.
Hong, J., O.M. Bhat, G. Li, S.K. Dempsey, Q. Zhang, J.K. Ritter, et al. 2019. Lysosomal regulation of extracellular vesicle excretion during d-ribose-induced NLRP3 inflammasome activation in podocytes. Biochimica et Biophysica Acta, Molecular Cell Research 1866: 849–860.
Huang, D., G. Li, Q. Zhang, O.M. Bhat, Y. Zou, J.K. Ritter, et al. 2021. Contribution of podocyte inflammatory exosome release to glomerular inflammation and sclerosis during hyperhomocysteinemia. Biochimica et Biophysica Acta, Molecular Basis of Disease 1867.
Li, G., D. Huang, N. Li, J.K. Ritter, and P.L. Li. 2021. Regulation of TRPML1 channel activity and inflammatory exosome release by endogenously produced reactive oxygen species in mouse podocytes. Redox biology 43.
Kajimoto, T., T. Okada, S. Miya, L. Zhang, and S. Nakamura. 2013. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nature communications 4: 2712.
Trajkovic, K., C. Hsu, S. Chiantia, L. Rajendran, D. Wenzel, F. Wieland, et al. 2008. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319: 1244–1247.
Yuyama, K., H. Sun, S. Mitsutake, and Y. Igarashi. 2012. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. The Journal of biological chemistry 287: 10977–10989.
Alvarez-Erviti, L., Y. Seow, A.H. Schapira, C. Gardiner, I.L. Sargent, M.J. Wood, et al. 2011. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiology of Diseases 42: 360–367.
Cui, Y., J. Luan, H. Li, X. Zhou, and J. Han. 2016. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Letters 590: 185–192.
Lee, M.J., J.R. Van Brocklyn, S. Thangada, C.H. Liu, A.R. Hand, R. Menzeleev, et al. 1998. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 279: 1552–1555.
Li, P.L., Y. Zhang, J.M. Abais, J.K. Ritter, and F. Zhang. 2013. Cyclic ADP-ribose and NAADP in vascular regulation and diseases. Messenger 2: 63–85.
Liebau, M.C., F. Braun, K. Hopker, C. Weitbrecht, V. Bartels, R.U. Muller, et al. 2013. Dysregulated autophagy contributes to podocyte damage in Fabry’s disease. PLoS One1 8.
Lorber, D. 2014. Importance of cardiovascular disease risk management in patients with type 2 diabetes mellitus. Diabetes, Metabolic Syndrome and Obesity 7: 169–183.
Boulanger, C.M., X. Loyer, P.E. Rautou, and N. Amabile. 2017. Extracellular vesicles in coronary artery disease. Nature Reviews. Cardiology 14: 259–272.
Chistiakov, D.A., A.N. Orekhov, and Y.V. Bobryshev. 2015. Extracellular vesicles and atherosclerotic disease. Cellular and molecular life sciences: CMLS 72: 2697–2708.
Hessvik, N.P., and A. Llorente. 2018. Current knowledge on exosome biogenesis and release. Cellular and Molecular Life Sciences 75: 193–208.
Li, G., D. Huang, J. Hong, O.M. Bhat, X. Yuan, and P.L. Li. 2019. Control of lysosomal TRPML1 channel activity and exosome release by acid ceramidase in mouse podocytes. American Journal of Physiology Cell Physiology 317: C481–C491.
Li, G., D. Huang, O.M. Bhat, J.L. Poklis, A. Zhang, Y. Zou, et al. 2020. Abnormal podocyte TRPML1 channel activity and exosome release in mice with podocyte-specific Asah1 gene deletion. Biochimica et biophysica acta Molecular and cell biology of lipids 1866.
Gupta, S., R. Natarajan, S.G. Payne, E.J. Studer, S. Spiegel, P. Dent, et al. 2004. Deoxycholic acid activates the c-Jun N-terminal kinase pathway via FAS receptor activation in primary hepatocytes. Role of acidic sphingomyelinase-mediated ceramide generation in FAS receptor activation. The Journal of biological chemistry 279: 5821–8.
Maric, I., J.P. Krieger, P. van der Velden, S. Borchers, M. Asker, M. Vujicic, et al. 2022. Sex and species differences in the development of diet-induced obesity and metabolic disturbances in rodents. Frontiers in nutrition 9.
Shah, B., K. Tombeau Cost, A. Fuller, C.S. Birken, and L.N. Anderson. 2020. Sex and gender differences in childhood obesity: Contributing to the research agenda. BMJ Nutrition, Prevention & Health 3: 387–390.
Li, G., J. Kidd, C. Kaspar, S. Dempsey, O.M. Bhat, S. Camus, et al. 2020. Podocytopathy and nephrotic syndrome in mice with podocyte-specific deletion of the Asah1 gene: Role of ceramide accumulation in glomeruli. The American journal of pathology 190: 1211–1223.
Huang, D., G. Li, O.M. Bhat, Y. Zou, N. Li, J.K. Ritter, et al. 2022. Exosome biogenesis and lysosome function determine podocyte exosome release and glomerular inflammatory response during hyperhomocysteinemia. The American journal of pathology 192: 43–55.
Koka, S., M. Xia, C. Zhang, Y. Zhang, P.L. Li, and K.M. Boini. 2019. Podocyte NLRP3 inflammasome activation and formation by adipokine visfatin. Cellular physiology and biochemistry: International journal of experimental cellular physiology, biochemistry, and pharmacology 53: 355–365.
Chen, Y., A.L. Pitzer, X. Li, P.L. Li, L. Wang, and Y. Zhang. 2015. Instigation of endothelial Nlrp3 inflammasome by adipokine visfatin promotes inter-endothelial junction disruption: Role of HMGB1. Journal of cellular and molecular medicine 19: 2715–2727.
Xia, M., C. Zhang, K.M. Boini, A.M. Thacker, and P.L. Li. 2011. Membrane raft-lysosome redox signalling platforms in coronary endothelial dysfunction induced by adipokine visfatin. Cardiovascular research 89: 401–409.
Boini, K.M., C. Zhang, M. Xia, W.Q. Han, C. Brimson, J.L. Poklis, et al. 2010. Visfatin-induced lipid raft redox signaling platforms and dysfunction in glomerular endothelial cells. Biochimica et biophysica acta 1801: 1294–1304.
Speakman, J.R. 2019. Use of high-fat diets to study rodent obesity as a model of human obesity. International journal of obesity 43: 1491–1492.
Tilg, H., and A.R. Moschen. 2008. Role of adiponectin and PBEF/visfatin as regulators of inflammation: Involvement in obesity-associated diseases. Clinical science 114: 275–288.
Hasegawa, M., R. Imamura, K. Motani, T. Nishiuchi, N. Matsumoto, T. Kinoshita, et al. 2009. Mechanism and repertoire of ASC-mediated gene expression. Journal of immunology 182: 7655–7662.
Tang, J., H. Yan, and S. Zhuang. 2012. Inflammation and oxidative stress in obesity-related glomerulopathy. International journal of nephrology 2012.
Mima, A., T. Yasuzawa, G.L. King, and S. Ueshima. 2018. Obesity-associated glomerular inflammation increases albuminuria without renal histological changes. FEBS Open Bio 8: 664–670.
Hou, X.X., H.R. Dong, L.J. Sun, M. Yang, H. Cheng, and Y.P. Chen. 2018. Purinergic 2X7 Receptor is involved in the podocyte damage of obesity-related glomerulopathy via activating nucleotide-binding and oligomerization domain-like receptor protein 3 inflammasome. Chinese medical journal 131: 2713–2725.
Xu, X., X. Huang, L. Zhang, X. Huang, Z. Qin, and F. Hua. 2021. Adiponectin protects obesity-related glomerulopathy by inhibiting ROS/NF-kappaB/NLRP3 inflammation pathway. BMC nephrology 22: 218.
Boini, K.M., C. Zhang, M. Xia, J.L. Poklis, and P.L. Li. 2010. Role of sphingolipid mediator ceramide in obesity and renal injury in mice fed a high-fat diet. The Journal of pharmacology and experimental therapeutics 334: 839–846.
Zhang, A.Y., F. Yi, S. Jin, M. Xia, Q.Z. Chen, E. Gulbins, et al. 2007. Acid sphingomyelinase and its redox amplification in formation of lipid raft redox signaling platforms in endothelial cells. Antioxidants & redox signaling 9: 817–828.
Evavold, C.L., J. Ruan, Y. Tan, S. Xia, H. Wu, and J.C. Kagan. 2018. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48 (35–44).
He, W.T., H. Wan, L. Hu, P. Chen, X. Wang, Z. Huang, et al. 2015. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell research 25: 1285–1298.
Carta, S., S. Tassi, I. Pettinati, L. Delfino, C.A. Dinarello, and A. Rubartelli. 2011. The rate of interleukin-1beta secretion in different myeloid cells varies with the extent of redox response to Toll-like receptor triggering. Journal of Biological Chemistry 286: 27069–27080.
Carta, S., F. Penco, R. Lavieri, A. Martini, C.A. Dinarello, M. Gattorno, et al. 2015. Cell stress increases ATP release in NLRP3 inflammasome-mediated autoinflammatory diseases, resulting in cytokine imbalance. Proceedings of the National Academy of Sciences USA 112: 2835–2840.
Liston, A., and S.L. Masters. 2017. Homeostasis-altering molecular processes as mechanisms of inflammasome activation. Nature Reviews Immunology 17: 208–214.
Yamagishi, R., F. Kamachi, M. Nakamura, S. Yamazaki, T. Kamiya, M. Takasugi, et al. 2022. Gasdermin D-mediated release of IL-33 from senescent hepatic stellate cells promotes obesity-associated hepatocellular carcinoma. Science immunology 7: eabl7209.
Rodriguez-Antonio, I., G.N. Lopez-Sanchez, M. Uribe, N.C. Chavez-Tapia, and N. Nuno-Lambarri. 2021. Role of the inflammasome, gasdermin D, and pyroptosis in non-alcoholic fatty liver disease. Journal of gastroenterology and hepatology 36: 2720–2727.
Xu, B., M. Jiang, Y. Chu, W. Wang, D. Chen, X. Li, et al. 2018. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. Journal of hepatology 68: 773–782.
Settembre, C., and A. Ballabio. 2014. Lysosomal adaptation: how the lysosome responds to external cues. Cold Spring Harbor perspectives in biology 6.
Xia, M., K.M. Boini, J.M. Abais, M. Xu, Y. Zhang, and P.L. Li. 2014. Endothelial NLRP3 inflammasome activation and enhanced neointima formation in mice by adipokine visfatin. The American journal of pathology 184: 1617–1628.
Funding
This study was supported by NIH grants DK054927 and DK120491.
Author information
Authors and Affiliations
Contributions
Conceptualization: Pin-Lan Li and Guangbi Li. Methodology: Dandan Huang, Yao Zou, Xiaoyuan Wu, and Guangbi Li. Formal analysis and investigation: Dandan Huang and Guangbi Li. Writing—original draft preparation: Dandan Huang and Guangbi Li. Writing—review and editing: Jason M. Kidd, Todd W.B. Gehr, and Pin-Lan Li. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, D., Kidd, J.M., Zou, Y. et al. Regulation of NLRP3 Inflammasome Activation and Inflammatory Exosome Release in Podocytes by Acid Sphingomyelinase During Obesity. Inflammation 46, 2037–2054 (2023). https://doi.org/10.1007/s10753-023-01861-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01861-y