Abstract
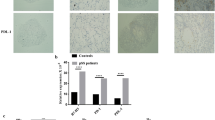
Primary Sjogren’s syndrome (pSS) is a systemic autoimmune disease that causes dysfunction of secretory glands and the specific pathogenesis is still unknown. The CXCL9, 10, 11/CXCR3 axis and G protein-coupled receptor kinase 2 (GRK2) involved in many inflammation and immunity processes. We used NOD/Ltj mice, a spontaneous SS animal model, to elucidate the pathological mechanism of CXCL9, 10, 11/CXCR3 axis promoting T lymphocyte migration by activating GRK2 in pSS. We found that CD4 + GRK2, Th17 + CXCR3 was apparently increased and Treg + CXCR3 was significantly decreased in the spleen of 4W NOD mice without sicca symptom compared to ICR mice (control group). The protein levels of IFN-γ, CXCL9, 10, 11 increased in submandibular gland (SG) tissue accompanied by obvious lymphocytic infiltration and Th17 cells overwhelmingly infiltrated relative to Treg cells at the sicca symptom occurs, and we found that the proportion of Th17 cells was increased, whereas that of Treg cells was decreased in spleen. In vitro, we used IFN-γ to stimulate human salivary gland epithelial cells (HSGECs) co-cultured with Jurkat cells, and the results showed that CXCL9, 10, 11 was increased by IFN-γ activating JAK2/STAT1 signal pathway and Jurkat cell migration increased with the raised of cell membrane GRK2 expression. HSGECs with tofacitinib or Jurkat cells with GRK2 siRNA can reduce the migration of Jurkat cells. The results indicate that CXCL9, 10, 11 significantly increased in SG tissue through IFN-γ stimulating HSGECs, and the CXCL9, 10, 11/CXCR3 axis contributes to the progress of pSS by activating GRK2 to promote T lymphocyte migration.






Similar content being viewed by others
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
References
Brito-Zerón, P., C. Baldini, H. Bootsma, S.J. Bowman, R. Jonsson, X. Mariette, K. Sivils, E. Theander, A. Tzioufas, and M. Ramos-Casals. 2016. Sjögren syndrome. Nature Reviews Disease Primers
Stefanski, A.L., C. Tomiak, U. Pleyer, T. Dietrich, G.R. Burmester, and T. Dorner. 2017. The Diagnosis and Treatment of Sjogren’s Syndrome. Deutsches Ärzteblatt International 114: 354–361.
Both, T., V.A.S.H. Dalm, P.M. van Hagen, and P.L.A. van Daele. 2017. Reviewing primary Sjögren’s syndrome: Beyond the dryness - From pathophysiology to diagnosis and treatment. International Journal of Medical Sciences 14: 191–200.
Yao, Y., J. Ma, C. Chang, T. Xu, C. Gao, M.E. Gershwin, and Z. Lian. 2021. Immunobiology of T Cells in Sjögren’s Syndrome. Clinical Reviews in Allergy & Immunology 60: 111–131.
Verstappen, G.M., S. Pringle, H. Bootsma, and F. Kroese. 2021. Epithelial-immune cell interplay in primary Sjogren syndrome salivary gland pathogenesis. Nature Reviews Rheumatology 17: 333–348.
Nikolov, N.P., and G.G. Illei. 2009. Pathogenesis of Sjögren’s syndrome. Current Opinion in Rheumatology 21: 465–470.
Ogawa, Y., E. Shimizu, and K. Tsubota. 2018. Interferons and Dry Eye in Sjögren’s Syndrome. International Journal of Molecular Sciences 19: 3548.
Van Raemdonck, K., P.E. Van den Steen, S. Liekens, J. Van Damme, and S. Struyf. 2015. CXCR3 ligands in disease and therapy. Cytokine & Growth Factor Reviews 26: 311–327.
Yoon, K.C., C.S. Park, I.C. You, H.J. Choi, K.H. Lee, S.K. Im, H.Y. Park, and S.C. Pflugfelder. 2010. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Investigative Ophthalmology & Visual Science 51: 643–650.
James, J.A., J.M. Guthridge, H. Chen, R. Lu, R.L. Bourn, K. Bean, M.E. Munroe, M. Smith, E. Chakravarty, A.N. Baer, G. Noaiseh, A. Parke, K. Boyle, L. Keyes-Elstein, A. Coca, T. Utset, M.C. Genovese, V. Pascual, P.J. Utz, V.M. Holers, K.D. Deane, K.L. Sivils, T. Aberle, D.J. Wallace, J. McNamara, N. Franchimont, and St. Clair, E.W. 2020. Unique Sjögren’s syndrome patient subsets defined by molecular features. Rheumatology 59: 860–868.
Antonelli, A., S.M. Ferrari, D. Giuggioli, E. Ferrannini, C. Ferri, and P. Fallahi. 2014. Chemokine (C–X–C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmunity Reviews 13: 272–280.
Panzer, U., G. Zahner, U. Wienberg, O.M. Steinmetz, A. Peters, J.E. Turner, H.J. Paust, G. Wolf, R.A.K. Stahl, and A. Schneider. 2008. 15-Deoxy- 12,14-prostaglandin J2 inhibits INF- -induced JAK/STAT1 signalling pathway activation and IP-10/CXCL10 expression in mesangial cells. Nephrology Dialysis Transplantation 23: 3776–3785.
Barrera, M., S. Aguilera, I. Castro, S. Matus, P. Carvajal, C. Molina, S. González, D. Jara, M. Hermoso, and M. González. 2021. Tofacitinib counteracts IL-6 overexpression induced by deficient autophagy: Implications in Sjögren’s syndrome. Rheumatology 60: 1951–1962.
Eiger, D.S., N. Boldizsar, C.C. Honeycutt, J. Gardner, S. Kirchner, C. Hicks, I. Choi, U. Pham, K. Zheng, A. Warman, J.S. Smith, J.Y. Zhang, and S. Rajagopal. 2022. Location bias contributes to functionally selective responses of biased CXCR3 agonists. Nature Communications 13.
Satarkar, D., and Patra, C. 2022. Evolution, Expression and Functional Analysis of CXCR3 in Neuronal and Cardiovascular Diseases: A Narrative Review. Frontiers in Cell and Developmental Biology 10.
Zhou, J., and Q. Yu. 2018. Disruption of CXCR3 function impedes the development of Sjögren’s syndrome-like xerostomia in non-obese diabetic mice. Laboratory Investigation 98: 620–628.
Allred, M., M.S. Chimenti, A.E. Ciecko, Y. Chen, and S.M. Lieberman. 2021. Characterization of Type I Interferon-Associated Chemokines and Cytokines in Lacrimal Glands of Nonobese Diabetic Mice. International Journal of Molecular Sciences 22: 3767.
Tokunaga, R., W. Zhang, M. Naseem, A. Puccini, M.D. Berger, S. Soni, M. McSkane, H. Baba, and H. Lenz. 2018. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation – A target for novel cancer therapy. Cancer Treatment Reviews 63: 40–47.
Palmer, C.B., G. D’Uonnolo, R. Luís, M. Meyrath, T. Uchański, A. Chevigné, and M. Szpakowska. 2022. Nanoluciferase-based complementation assay for systematic profiling of GPCR-GRK interactions. Methods in Cell Biology 169: 309–321.
Smith, J.S., P. Alagesan, N.K. Desai, T.F. Pack, J. Wu, A. Inoue, N.J. Freedman, and S. Rajagopal. 2017. C-X-C Motif Chemokine Receptor 3 Splice Variants Differentially Activate Beta-Arrestins to Regulate Downstream Signaling Pathways. Molecular Pharmacology 92: 136–150.
Scuron, M.D., B. Fay, J. Oliver, and P. Smith. 2019. Spontaneous Model of Sjögren’s Syndrome in NOD Mice. Current Protocols in Pharmacology 86.
Bautista-Vargas, M., A.J. Vivas, and G.J. Tobón. 2020. Minor salivary gland biopsy: Its role in the classification and prognosis of Sjögren’s syndrome. Autoimmunity Reviews 19: 102690.
Nocturne, G., and X. Mariette. 2013. Advances in understanding the pathogenesis of primary Sjogren’s syndrome. Nature Reviews Rheumatology 9: 544–556.
Sarkar, I., R. Davies, A.K. Aarebrot, S.M. Solberg, A. Petrovic, A.M. Joshi, B. Bergum, J.G. Brun, D. Hammenfors, R. Jonsson, and S. Appel. 2022. Aberrant signaling of immune cells in Sjögren’s syndrome patient subgroups upon interferon stimulation. Frontiers in Immunology 13.
Grange, L., E. Chalayer, D. Boutboul, S. Paul, L. Galicier, B. Gramont, and M. Killian. 2022. TAFRO syndrome: A severe manifestation of Sjogren’s syndrome? A systematic review. Autoimmun Rev 21: 103137.
Bombardieri, M., O.D. Argyropoulou, F. Ferro, R. Coleby, E. Pontarini, G. Governato, D. Lucchesi, G. Fulvio, A.G. Tzioufas, and C. Baldini. 2020. One year in review 2020: Pathogenesis of primary Sjogren’s syndrome. Clinical and Experimental Rheumatology 38 (Suppl 126): 3–9.
Negrini, S., G. Emmi, M. Greco, M. Borro, F. Sardanelli, G. Murdaca, F. Indiveri, and F. Puppo. 2022. Sjögren’s syndrome: A systemic autoimmune disease. Clinical and Experimental Medicine 22: 9–25.
Nezos, A., F. Gravani, A. Tassidou, E.K. Kapsogeorgou, M. Voulgarelis, M. Koutsilieris, M.K. Crow, and C.P. Mavragani. 2015. Type I and II interferon signatures in Sjogren’s syndrome pathogenesis: Contributions in distinct clinical phenotypes and Sjogren’s related lymphomagenesis. Journal of Autoimmunity 63: 47–58.
Hall, J.C., A.N. Baer, A.A. Shah, L.A. Criswell, C.H. Shiboski, A. Rosen, and L. Casciola-Rosen. 2015. Molecular Subsetting of Interferon Pathways in Sjögren’s Syndrome. Arthritis & Rheumatology 67: 2437–2446.
Hall, J.C., L. Casciola-Rosen, A.E. Berger, E.K. Kapsogeorgou, C. Cheadle, A.G. Tzioufas, A.N. Baer, and A. Rosen. 2012. Precise probes of type II interferon activity define the origin of interferon signatures in target tissues in rheumatic diseases. Proceedings of the National Academy of Sciences 109: 17609–17614.
Voynova, E., F. Lefebvre, A. Qadri, and S. Muller. 2020. Correction of autophagy impairment inhibits pathology in the NOD.H-2h4 mouse model of primary Sjögren’s syndrome. Journal of Autoimmunity 108, 102418.
Yu, S., L. Sun, Y. Jiao, and L.T.O. Lee. 2018. The Role of G Protein-coupled Receptor Kinases in Cancer. International Journal of Biological Sciences 14: 189–203.
Jiménez-Sainz, M.C., C. Murga, A. Kavelaars, M. Jurado-Pueyo, B.F. Krakstad, C.J. Heijnen, F.J. Mayor, and A.M. Aragay. 2006. G protein-coupled receptor kinase 2 negatively regulates chemokine signaling at a level downstream from G protein subunits. Molecular Biology of the Cell 17: 25–31.
Liampas, A., K. Parperis, M.F. Erotocritou, A. Nteveros, M. Papadopoulou, C. Moschovos, M. Akil, S. Coaccioli, G.M. Hadjigeorgiou, M. Hadjivassiliou, and P. Zis. 2022. Primary Sjögren syndrome‐related peripheral neuropathy: A systematic review and meta‐analysis. European Journal of Neurology.
Zhao, J., Q. An, X. Zhu, B. Yang, X. Gao, Y. Niu, L. Zhang, K. Xu, and D. Ma. 2022. Research status and future prospects of extracellular vesicles in primary Sjögren’s syndrome. Stem Cell Research and Therapy 13.
Funding
This study was supported by the National Nature Science Foundation of China (Nos. 81973332 and 81302784), Research Fund of Anhui Institute of translational medicine (2021zhyx-C24), Doctoral Research Grant Fund of Anhui Medical University (XJ201938), 2021 Anhui Medical University Discipline Construction Project (Surgery and Pharmacology Co-construction Project), and 2022 Anhui Provincial Natural Science Foundation (2208085MH283).
Author information
Authors and Affiliations
Contributions
JZ, XZ, XJS, YQL, QWT, and DQC conducted the study and analyzed the data, JZ, NL, and HXW provided expertise for design of the study and drafted the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Zhang, X., Shi, X. et al. CXCL9, 10, 11/CXCR3 Axis Contributes to the Progress of Primary Sjogren’s Syndrome by Activating GRK2 to Promote T Lymphocyte Migration. Inflammation 46, 1047–1060 (2023). https://doi.org/10.1007/s10753-023-01791-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01791-9