Abstract
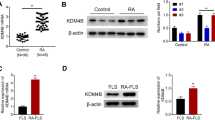
Rheumatoid arthritis (RA) is a chronic autoimmune disease that can lead to synovial inflammation, pannus formation, cartilage damage, bone destruction, and ultimate disability. Fibroblast-like synoviocytes (FLS) are involved in the pathogenetic mechanism of RA. Cdc37 (cell division cycle protein 37) is regarded as a molecular chaperone involved in various physiological processes such as cell cycle progression, cell proliferation, cell signal transduction, tumorigenesis, and progression. However, the precise role of Cdc37 in the pathogenesis of rheumatoid arthritis (RA) remains uncertain. In our study, we found that Cdc37 expression was upregulated in human rheumatoid synovia in contrast with the normal group. Interestingly, Cdc37 activated the ERK pathway to promote RA-FLS proliferation and migration in vitro. Ultimately, in vivo experiments revealed that silencing of Cdc37 alleviated ankle swelling and cartilage destruction and validated the ERK signaling pathways in vitro findings. Collectively, we demonstrate that Cdc37 promotes the proliferation and migration of RA-FLS by activation of ERK signaling pathways and finally aggravates the progression of RA. These data indicated that Cdc37 may be a novel target for the treatment of RA.






Similar content being viewed by others
Availability of Data and Materials
Data are available from the corresponding author on reasonable request.
References
Niu, Y., Q. Dong, and R. Li. 2017. Matrine regulates Th1/Th2 cytokine responses in rheumatoid arthritis by attenuating the NF-kappaB signaling. Cell Biology International 41: 611–621.
Agere, S.A., N. Akhtar, J.M. Watson, and S. Ahmed. 2017. RANTES/CCL5 induces collagen degradation by activating MMP-1 and MMP-13 expression in human rheumatoid arthritis synovial fibroblasts. Frontiers in Immunology 8: 1341.
Feldmann, M., F.M. Brennan, and R.N. Maini. 1996. Rheumatoid arthritis. Cell 85: 307–310.
Li, C.H., L.L. Xu, J.X. Zhao, L. Sun, Z.Q. Yao, X.L. Deng, R. Liu, L. Yang, R. Xing, and X.Y. Liu. 2016. CXCL16 upregulates RANKL expression in rheumatoid arthritis synovial fibroblasts through the JAK2/STAT3 and p38/MAPK signaling pathway. Inflammation Research 65: 193–202.
Liu, Y., Y.F. Pan, Y.Q. Xue, L.K. Fang, X.H. Guo, X. Guo, M. Liu, B.Y. Mo, M.R. Yang, F. Liu, et al. 2018. uPAR promotes tumor-like biologic behaviors of fibroblast-like synoviocytes through PI3K/Akt signaling pathway in patients with rheumatoid arthritis. Cellular & Molecular Immunology 15: 171–181.
Huber, L.C., O. Distler, I. Tarner, R.E. Gay, S. Gay, and T. Pap. 2006. Synovial fibroblasts: Key players in rheumatoid arthritis. Rheumatology (Oxford) 45: 669–675.
Okamoto, H., K. Shidara, D. Hoshi, and N. Kamatani. 2007. Anti-arthritis effects of vitamin K(2) (menaquinone-4)–a new potential therapeutic strategy for rheumatoid arthritis. FEBS Journal 274: 4588–4594.
Lee, H.S., S.J. Woo, H.W. Koh, S.O. Ka, L. Zhou, K.Y. Jang, H.S. Lim, H.O. Kim, S.I. Lee, and B.H. Park. 2014. Regulation of apoptosis and inflammatory responses by insulin-like growth factor binding protein 3 in fibroblast-like synoviocytes and experimental animal models of rheumatoid arthritis. Arthritis & Rhematology 66: 863–873.
Bartok, B., and G.S. Firestein. 2010. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunological Reviews 233: 233–255.
Nam, E.J., J.H. Kang, S. Sung, K.H. Sa, K.H. Kim, J.S. Seo, J.H. Kim, S.W. Han, I.S. Kim, and Y.M. Kang. 2013. A matrix metalloproteinase 1-cleavable composite peptide derived from transforming growth factor beta-inducible gene h3 potently inhibits collagen-induced arthritis. Arthritis and Rheumatism 65: 1753–1763.
Verba, K.A., R.Y. Wang, A. Arakawa, Y. Liu, M. Shirouzu, S. Yokoyama, and D.A. Agard. 2016. Atomic structure of Hsp90-Cdc37-Cdk4 reveals that Hsp90 traps and stabilizes an unfolded kinase. Science 352: 1542–1547.
Verba, K.A., and D.A. Agard. 2017. How Hsp90 and Cdc37 lubricate kinase molecular switches. Trends in Biochemical Sciences 42: 799–811.
Smith, J.R., P.A. Clarke, E. de Billy, and P. Workman. 2009. Silencing the cochaperone CDC37 destabilizes kinase clients and sensitizes cancer cells to HSP90 inhibitors. Oncogene 28: 157–169.
Gray, P.J., Jr., M.A. Stevenson, and S.K. Calderwood. 2007. Targeting Cdc37 inhibits multiple signaling pathways and induces growth arrest in prostate cancer cells. Cancer Research 67: 11942–11950.
El Hamidieh, A., N. Grammatikakis, and E. Patsavoudi. 2012. Cell surface Cdc37 participates in extracellular HSP90 mediated cancer cell invasion. PLoS ONE 7: e42722.
Wang, Z., W. Wei, C.K. Sun, M.S. Chua, and S. So. 2015. Suppressing the CDC37 cochaperone in hepatocellular carcinoma cells inhibits cell cycle progression and cell growth. Liver International 35: 1403–1415.
Schwarze, S.R., V.X. Fu, and D.F. Jarrard. 2003. Cdc37 enhances proliferation and is necessary for normal human prostate epithelial cell survival. Cancer Research 63: 4614–4619.
Ota, A., and Y. Wang. 2012. Cdc37/Hsp90 protein-mediated regulation of IRE1alpha protein activity in endoplasmic reticulum stress response and insulin synthesis in INS-1 cells. Journal of Biological Chemistry 287: 6266–6274.
Liu, Y., S. Wang, D. Ding, Z. Yu, W. Sun, and Y. Wang. 2018. Up-regulation of Cdc37 contributes to Schwann cell proliferation and migration after sciatic nerve crush. Neurochemical Research 43: 1182–1190.
Zhang, H.G., Y. Wang, J.F. Xie, X. Liang, D. Liu, P. Yang, H.C. Hsu, R.B. Ray, and J.D. Mountz. 2001. Regulation of tumor necrosis factor alpha-mediated apoptosis of rheumatoid arthritis synovial fibroblasts by the protein kinase Akt. Arthritis and Rheumatism 44: 1555–1567.
King, D., D. Yeomanson, and H.E. Bryant. 2015. PI3King the lock: Targeting the PI3K/Akt/mTOR pathway as a novel therapeutic strategy in neuroblastoma. Journal of Pediatric Hematology/oncology 37: 245–251.
Chen, B.C., and W.W. Lin. 2001. PKC- and ERK-dependent activation of I kappa B kinase by lipopolysaccharide in macrophages: Enhancement by P2Y receptor-mediated CaMK activation. British Journal of Pharmacology 134: 1055–1065.
Hsing, C.H., M.C. Lin, P.C. Choi, W.C. Huang, J.I. Kai, C.C. Tsai, Y.L. Cheng, C.Y. Hsieh, C.Y. Wang, Y.P. Chang, et al. 2011. Anesthetic propofol reduces endotoxic inflammation by inhibiting reactive oxygen species-regulated Akt/IKKbeta/NF-kappaB signaling. PLoS ONE 6: e17598.
Li, D.W., X.T. Wang, B.C. Mu, D.Q. Dou, and T.G. Kang. 2021. Effects of hydroxysafflor yellow A on rats with collagen-induced arthritis. Biochemical and Biophysical Research Communications 570: 26–34.
Yu, X., J. Zhou, F. Zhao, X. Liu, Y. Mao, L. Diao, C. Wen, and M. Liu. 2021. Tomatidine suppresses the destructive behaviors of fibroblast-like synoviocytes and ameliorates type II collagen-induced arthritis in rats. Frontiers in Pharmacology 12: 670707.
Liu, C., A. Pan, X. Chen, J. Tu, X. Xia, and L. Sun. 2019. MiR-5571-3p and miR-135b-5p, derived from analyses of microRNA profile sequencing, correlate with increased disease risk and activity of rheumatoid arthritis. Clinical Rheumatology 38: 1753–1765.
Fan, W., Z. Xu, S. Liang, S. Zuo, C. Bian, X. Gao, Y. Qin, and J. Wu. 2021. MLL3 inhibits apoptosis of rheumatoid arthritis fibroblast-like synoviocytes and promotes secretion of inflammatory factors by activating CCL2 and the NF-kappaB pathway. Inflammation 44: 1803–1814.
Li, G., Y. Liu, F. Meng, Z. Xia, X. Wu, Y. Fang, C. Zhang, Y. Zhang, and D. Liu. 2019. LncRNA MEG3 inhibits rheumatoid arthritis through miR-141 and inactivation of AKT/mTOR signalling pathway. Journal of Cellular and Molecular Medicine 23: 7116–7120.
Philippe, L., G. Alsaleh, A. Pichot, E. Ostermann, G. Zuber, B. Frisch, J. Sibilia, S. Pfeffer, S. Bahram, D. Wachsmann, and P. Georgel. 2013. MiR-20a regulates ASK1 expression and TLR4-dependent cytokine release in rheumatoid fibroblast-like synoviocytes. Annals of the Rheumatic Diseases 72: 1071–1079.
Zhang, T., H. Li, J. Shi, S. Li, M. Li, L. Zhang, L. Zheng, D. Zheng, F. Tang, X. Zhang, et al. 2016. p53 predominantly regulates IL-6 production and suppresses synovial inflammation in fibroblast-like synoviocytes and adjuvant-induced arthritis. Arthritis Research & Therapy 18: 271.
Liu, F.L., L.H. Lin, H.K. Sytwu, and D.M. Chang. 2010. GDF-5 is suppressed by IL-1beta and enhances TGF-beta3-mediated chondrogenic differentiation in human rheumatoid fibroblast-like synoviocytes. Experimental and Molecular Pathology 88: 163–170.
Qiu, H., S. Sun, X. Ma, C. Cui, G. Chen, Z. Liu, H. Li, and M. Liu M. 2018. Jatrorrhizine hydrochloride suppresses proliferation, migration, and secretion of synoviocytes in vitro and ameliorates rat models of rheumatoid arthritis in vivo. Internation Journal of Molecular Sciences 19.
Lee, J.W., J. Lee, S.H. Um, and E.Y. Moon. 2017. Synovial cell death is regulated by TNF-alpha-induced expression of B-cell activating factor through an ERK-dependent increase in hypoxia-inducible factor-1alpha. Cell Death & Disease 8: e2727.
Huang, C.C., C.H. Chiou, S.C. Liu, S.L. Hu, C.M. Su, C.H. Tsai, and C.H. Tang. 2019. Melatonin attenuates TNF-alpha and IL-1beta expression in synovial fibroblasts and diminishes cartilage degradation: Implications for the treatment of rheumatoid arthritis. Journal of Pineal Research 66: e12560.
Pinto, L.G., J. Talbot, R.S. Peres, R.F. Franca, S.H. Ferreira, B. Ryffel, J.C. Aves-Filho, F. Figueiredo, T.M. Cunha, and F.Q. Cunha. 2015. Joint production of IL-22 participates in the initial phase of antigen-induced arthritis through IL-1beta production. Arthritis Research & Therapy 17: 235.
Sparks, J.A. 2019. Rheumatoid Arthritis. Annals of Intern Medicine 170: ITC1-ITC16.
Krasselt, M., and C. Baerwald. 2019. Celecoxib for the treatment of musculoskeletal arthritis. Expert Opinion on Pharmacotherapy 20: 1689–1702.
Wang, W., H. Zhou, and L. Liu. 2018. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. European Journal of Medicinal Chemistry 158: 502–516.
Lin, Y.J., M. Anzaghe, and S. Schulke. 2020. Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells 9.
Alfaro-Lara, R., H.F. Espinosa-Ortega, and C.A. Arce-Salinas. 2019. Precis study group apbtDoIMHCSdP: Systematic review and meta-analysis of the efficacy and safety of leflunomide and methotrexate in the treatment of rheumatoid arthritis. Reumatol Clin (Engl Ed) 15: 133–139.
Aletaha, D., and J.S. Smolen. 2018. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 320: 1360–1372.
Pappas, D.A., G. St John, C.J. Etzel, S. Fiore, T. Blachley, T. Kimura, R. Punekar, K. Emeanuru, J. Choi, S. Boklage, and J.M. Kremer. 2021. Comparative effectiveness of first-line tumour necrosis factor inhibitor versus non-tumour necrosis factor inhibitor biologics and targeted synthetic agents in patients with rheumatoid arthritis: Results from a large US registry study. Annals of the Rheumatic Diseases 80: 96–102.
Nygaard, G., and G.S. Firestein. 2020. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nature Reviews Rheumatology 16: 316–333.
Lee, C.W., Y.C. Kwon, Y. Lee, M.Y. Park, and K.M. Choe. 2019. cdc37 is essential for JNK pathway activation and wound closure in Drosophila. Molecular Biology of the Cell 30: 2651–2658.
Eguchi, T., T.L. Prince, M.T. Tran, C. Sogawa, B.J. Lang, and S.K. Calderwood. 2019. MZF1 and SCAND1 reciprocally regulate CDC37 gene expression in prostate cancer. Cancers (Basel) 11.
Calvisi, D.F., R.M. Pascale, and F. Feo. 2007. Dissection of signal transduction pathways as a tool for the development of targeted therapies of hepatocellular carcinoma. Reviews on Recent Clinical Trials 2: 217–236.
Umar, S., K. Palasiewicz, K. Van Raemdonck, M.V. Volin, B. Romay, I. Ahmad, C. Tetali, N. Sweiss, M.A. Amin, R.K. Zomorrodi, and S. Shahrara. 2021. CCL25 and CCR9 is a unique pathway that potentiates pannus formation by remodeling RA macrophages into mature osteoclasts. European Journal of Immunology 51: 903–914.
Funding
This work was supported by A Project of Nantong Science and Technology Program (JC2020015) and the Research Innovation Program for College Graduates of Jiangsu Province (KYCX20-2800).
Author information
Authors and Affiliations
Contributions
Weiwei Sun and Xingxing Mao performed the experiments. Weijie Wu and Yunyi Nan collected and analyzed the data. Hua Xu and Youhua Wang performed joint surgeries and provided synovial tissue. Weiwei Sun and Weijie Wu prepared the manuscript. Chunxiang Xu contributed to the conception of the study. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
This study was approved by the institutional medical ethics committee of the Affiliated Hospital of Nantong University. Informed consent was acquired from all patients prior to surgery.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Weiwei Sun, Weijie Wu, and Xingxing Mao contributed equally and should be regarded as co-first authors.
Chunxiang Xu, Youhua Wang, and Hua Xu contributed equally and should be regarded as co-corresponding authors.
Supplementary Information
Below is the link to the electronic supplementary material.
10753_2023_1789_MOESM2_ESM.tif
Fig. S2 RA-FLS were transfected with siRNA#2 and then stimulated by IL-1β or Tnfα (10ng/ml, 24 hours). The protein expression levels of p-ERK and ERK were measured by western blot. Experiments were repeated for three times, * compare with Con, P<0.05 (TIF 1876 KB)
10753_2023_1789_MOESM4_ESM.tif
Fig. S4 H&E stained histological tissues of the main organs (heart, liver, spleen, lung, kidney) were collected from the different groups (TIF 144096 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, W., Mao, X., Wu, W. et al. Inhibition of Cdc37 Ameliorates Arthritis in Collagen-Induced Arthritis Rats by Inhibiting Synoviocyte Proliferation and Migration Through the ERK Pathway. Inflammation 46, 1022–1035 (2023). https://doi.org/10.1007/s10753-023-01789-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01789-3