Abstract
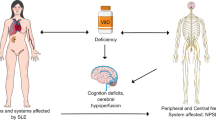
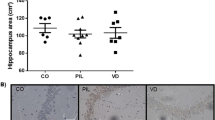
Neuropsychiatric systemic lupus erythematosus (NPSLE) is a serious complication of systemic lupus erythematosus (SLE) involving the nervous system with high morbidity and mortality. A key hypothesis in NPSLE is that a disrupted barrier allows autoantibodies and immune components of peripheral blood to penetrate into the central nervous system (CNS), resulting in inflammation and damage. The blood cerebrospinal fluid barrier (BCSFB), which consists of the choroid plexus and the hypothalamic tanycytes, has long been regarded as an immunological sanctuary site. 1,25-Dihydroxyvitamin D3 [1,25-(OH)2D3] is the active form of vitamin D, which plays multiple roles in inflammation and immunoregulation. In this study, we investigated the possible protective effects of 1,25-dihydroxyvitamin D3 against BCSFB dysfunction in NPSLE in MRL/lpr mice and explored the mechanism by which 1,25-dihydroxyvitamin D3 inhibits the progression of NPSLE. In this study, we found that supplementation with 1,25-dihydroxyvitamin D3 markedly improved serological and immunological indices, delayed inflammatory infiltration, delayed neuronal deformation, and upregulated the expression of brain-derived neurotrophic factor (BDNF) proteins in the brain. Furthermore, 1,25-dihydroxyvitamin D3 downregulated proinflammatory cytokines such as nuclear factor kappa-B (NF-κB) and tumor necrosis factor-α (TNF-α) by activating peroxisome proliferator-activated receptor γ (PPARγ), and it reduced the expression of the TGF-β/Smad signaling pathway. Our findings demonstrate that 1,25-dihydroxyvitamin D3 delayed cell infiltration into the choroid plexus and decreased markers suggestive of cognitive decline in MRL/lpr mice, and the mechanism may be related to protection against BCSFB disruption through activation of the anti-inflammatory PPARγ/NF-κB/TNF-α pathway as well as upregulation of BDNF and inhibition of the TGF-β/Smad signaling pathway. These findings provide a novel direction for the study of NPSLE.









Similar content being viewed by others
DATA AVAILABILITY
The datasets during the current study are available from the corresponding author on reasonable request.
References
Kiriakidou, M., and C.L. Ching. 2020. Systemic lupus erythematosus. Annals of Internal Medicine 172(11):ITC81-ITC96.
Hanly, J.G., E. Kozora, S.D. Beyea, et al. 2019. Review: Nervous system disease in systemic lupus erythematosus: Current status and future directions. Arthritis & Rhematology 71 (1): 33–42.
Tanaka, Y. 2019. Neuropsychiatric systemic lupus erythematosus. Brain and Nerve 71 (5): 445–458.
Moore, E., M.W. Huang, and C. Putterman. 2020. Advances in the diagnosis, pathogenesis and treatment of neuropsychiatric systemic lupus erythematosus. Current Opinion in Rheumatology 32 (2): 152–158.
Schwartz, N., A.D. Stock, and C. Putterman. 2019. Neuropsychiatric lupus: New mechanistic insights and future treatment directions. Nature Reviews Rheumatology 15 (3): 137–152.
Lee, S.M., A.H. Carlson, M. Onal, et al. 2019. A control region near the fibroblast growth factor 23 gene mediates response to phosphate, 1,25(OH)2D3, and LPS in vivo. Endocrinology 160 (12): 2877–2891.
Park, C.Y., T.Y. Kim, J.S. Yoo, et al. 2020. Effects of 1,25-dihydroxyvitamin D3 on the inflammatory responses of stromal vascular cells and adipocytes from lean and obese mice. Nutrients 12 (2): 364.
Franco, A.S., T.Q. Freitas, W.M. Bernardo, et al. 2017. Vitamin D supplementation and disease activity in patients with immune-mediated rheumatic diseases: A systematic review and meta-analysis. Medicine (Baltimore) 96 (23): e7024.
Wang, X., and Y. Xia. 2019. Anti-double stranded DNA antibodies: Origin, pathogenicity, and targeted therapies. Frontiers in Immunology 10: 1667.
Petri, M.A., J. Conklin, T. O’Malley, et al. 2019. Platelet-bound C4d, low C3 and lupus anticoagulant associate with thrombosis in SLE. Lupus Sci Med 6 (1): e000318.
Dunn, J.F., and A.M. Isaacs. 2021. The impact of hypoxia on blood-brain, blood-CSF, and CSF-brain barriers. Journal of Applied Physiology (1985) 131(3):977–985.
Gelb, S., A.D. Stock, S. Anzi, et al. 2018. Mechanisms of neuropsychiatric lupus: The relative roles of the blood-cerebrospinal fluid barrier versus blood-brain barrier. Journal of Autoimmunity 91: 34–44.
Villapol, S. 2018. Roles of peroxisome proliferator-activated receptor gamma on brain and peripheral inflammation. Cellular and Molecular Neurobiology 38 (1): 121–132.
Xie, Z., L. Huang, B. Enkhjargal, et al. 2018. Recombinant Netrin-1 binding UNC5B receptor attenuates neuroinflammation and brain injury via PPARγ/NFκB signaling pathway after subarachnoid hemorrhage in rats. Brain, Behavior, and Immunity 69: 190–202.
Guo, T., Y. Wang, Y. Guo, et al. 2018. 1, 25–D3 protects from cerebral ischemia by maintaining BBB permeability via PPAR-γ activation. Frontiers in Cellular Neuroscience 12: 480.
Palasz, E., A. Wysocka, A. Gasiorowska, et al. 2020. BDNF as a promising therapeutic agent in Parkinson’s disease. International Journal of Molecular Sciences 21 (3): 1170.
Vander Ark, A., J. Cao, and X. Li. 2018. TGF-β receptors: In and beyond TGF-β signaling. Cellular Signalling 52: 112–120.
Qu, S., L. Yang, and Z. Liu. 2020. MicroRNA-194 reduces inflammatory response and human dermal microvascular endothelial cells permeability through suppression of TGF-β/SMAD pathway by inhibiting THBS1 in chronic idiopathic urticaria. Journal of Cellular Biochemistry 121 (1): 111–124.
Hiew, L.F., C.H. Poon, H.Z. You, et al. 2021. TGF-β/Smad signalling in neurogenesis: Implications for neuropsychiatric diseases. Cell 10 (6): 1382.
Andrews, B.S., R.A. Eisenberg, and A.N. Theofilopoulos. 1978. Spontaneous murine lupus-like syndromes: Clinical and immunopathological manifestations in several strains. Journal of Experimental Medicine 148 (5): 1198–1215.
Li, Y., A.R. Eskelund, H. Zhou, et al. 2015. Behavioral deficits are accompanied by immunological and neurochemical changes in a mouse model for neuropsychiatric lupus (NP-SLE). International Journal of Molecular Sciences 16 (7): 15150–15171.
Szechtman, H., and Saki´c B, Denburg JA. 1997. Behaviour of MRL mice: An animal model of disturbed behaviour in systemic autoimmune disease. Lupus 6: 223–229.
Stielke, S., G. Keilhoff, E. Kirches, et al. 2012. Adhesion molecule expression precedes brain damages of lupus-prone mice and correlates with kidney pathology. Neuroimmunology 252: 24–32.
Nystad, A.E., R.R. Lereim, S. Wergeland, et al. 2020. Fingolimod downregulates brain sphingosine-1-phosphate receptor 1 levels but does not promote remyelination or neuroprotection in the cuprizone model. Neuroimmunology 339: 577091
Mike, E.V., H.M. Makinde, M. Gulinello, et al. 2018. Lipocalin-2 is a pathogenic determinant and biomarker of neuropsychiatric lupus. Autoimmun 96: 59–73.
Chen, E.Y., C.M. Tan, Y. Kou, et al. 2013. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14 (1): 128.
Kuleshov, M.V., M.R. Jones, A.D. Rouillard, et al. 2016. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Research 44 (W1): W90–W97.
Chen, X., T.W. Laudeman, P.J. Rushton, et al. 2007. CGKB: An annotation knowledge base for cowpea (Vigna unguiculata L.) methylation filtered genomic genespace sequences. BMC Bioinformatics 8:129.
Xie, Z., A. Bailey, M.V. Kuleshov, et al. 2021. Gene set knowledge discovery with Enrichr. Curr Protoc 1 (3): e90.
Wang, J., Y. Liu, X. Cheng, et al. 2017. The effects of LW-AFC on the hippocampal transcriptome in senescence-accelerated mouse prone 8 strain, a mouse model of Alzheimer’s disease. Journal of Alzheimer’s Disease 57 (1): 227–240.
Qiao, X., H. Wang, L. Lu, et al. 2021. Hippocampal microglia CD40 mediates NPSLE cognitive dysfunction in mice. Journal of Neuroimmunology 357: 577620.
Sato, S., J. Temmoku, Y. Fujita, et al. 2020. Autoantibodies associated with neuropsychiatric systemic lupus erythematosus: The quest for symptom-specific biomarkers. Fukushima Journal of Medical Sciences 66 (1): 1–9.
Wen, J., J. Doerner, K. Weidenheim, et al. 2015. TNF-like weak inducer of apoptosis promotes blood brain barrier disruption and increases neuronal cell death in MRL/lpr mice. Journal of Autoimmunity 60: 40–50.
Ramsey-Goldman, R., J. Li, T. Dervieux, et al. 2017. Cell-bound complement activation products in SLE. Lupus Science and Medicine 4(1): e000236
Fanouriakis, A., D.T. Boumpas, and G.K. Bertsias. 2013. Pathogenesis and treatment of CNS lupus. Current Opinion in Rheumatology 25 (5): 577–583.
Lu, L., H. Wang, X. Liu, et al. 2021. Pyruvate kinase isoform M2 impairs cognition in systemic lupus erythematosus by promoting microglial synaptic pruning via the β-catenin signaling pathway. Neuroinflammation 18 (1): 229.
Duarte-Delgado, N.P., G. Vásquez, and B.L. Ortiz-Reyes. 2019. Blood-brain barrier disruption and neuroinflammation as pathophysiological mechanisms of the diffuse manifestations of neuropsychiatric systemic lupus erythematosus. Autoimmunity Reviews 18 (4): 426–432.
Wang, J., Y. Liu, X. Cheng, et al. 2017. The effects of LW-AFC on the hippocampal transcriptome in senescence-accelerated mouse prone 8 strain, a mouse model of Alzheimer’s disease. Alzheimers Dis 57: 227–240.
Bărbulescu, A.L., R.E. Sandu, A.F. Vreju, et al. 2019. Neuroinflammation in systemic lupus erythematosus - A review. Romanian Journal of Morphology and Embryology 60 (3): 781–786.
Faust, T.W., E.H. Chang, C. Kowal, et al. 2010. Neurotoxic lupus autoantibodies alter brain function through two distinct mechanisms. Proceedings of the National Academy of Sciences USA 107 (43): 18569–18574.
DeGiorgio, L.A., K.N. Konstantinov, S.C. Lee, et al. 2001. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nature Medicine 7 (11): 1189–1193.
Kowal, C., L.A. Degiorgio, J.Y. Lee, et al. 2006. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proceedings of the National Academy of Sciences USA 103 (52): 19854–19859.
Stock, A.D., S. Gelb, O. Pasternak, et al. 2017. The blood brain barrier and neuropsychiatric lupus: New perspectives in light of advances in understanding the neuroimmune interface. Autoimmunity Reviews 16 (6): 612–619.
Erickson, M.A., and W.A. Banks. 2019. Age-associated changes in the immune system and blood-brain barrier functions. International Journal of Molecular Sciences 20 (7): 1632.
Liu, L., and X. Liu. 2019. Contributions of drug transporters to blood-brain barriers. Advances in Experimental Medicine and Biology 1141: 407–466.
Erb, U., C. Schwerk, H. Schroten, et al. 2020. Review of functional in vitro models of the blood-cerebrospinal fluid barrier in leukaemia research. Journal of Neuroscience Methods 329: 108478.
Solár, P., A. Zamani, L. Kubíčková, et al. 2020. Choroid plexus and the blood-cerebrospinal fluid barrier in disease. Fluids Barriers CNS 17 (1): 35.
Zanotto, C., F. Simão, M.S. Gasparin, et al. 2017. Exendin-4 reverses biochemical and functional alterations in the blood-brain and blood-CSF barriers in diabetic rats. Molecular Neurobiology 54 (3): 2154–2166.
Abdelrahman, M., A. Sivarajah, and C. Thiemermann. 2005. Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion injury, inflammation and shock. Cardiovascular Research 65: 772–781.
Akiyama, T.E., P.T. Meinke, and J.P. Berger. 2005. PPAR ligands: Potential therapies for metabolic syndrome. Current Diabetes Reports 5: 45–52.
Bernardo, A., and L. Minghetti. 2006. PPAR-gamma agonists as regulators of microglial activation and brain inflammation. Current Pharmaceutical Design 12: 93–109.
He, X., W. Liu, M. Shi, et al. 2017. Docosahexaenoic acid attenuates LPS-stimulated inflammatory response by regulating the PPARγ/NF-κB pathways in primary bovine mammary epithelial cells. Research in Veterinary Science 112: 7–12.
Zhang, X., M. Zhou, Y. Guo, et al. 2015. 1,25-Dihydroxyvitamin D3 promotes high glucose-induced M1 macrophage switching to M2 via the VDR-PPARγ signaling pathway. BioMed Research International 2015: 157834.
Kowiański, P., G. Lietzau, E. Czuba, et al. 2018. BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cellular and Molecular Neurobiology 38 (3): 579–593.
Hiew, L.F., C.H. Poon, H.Z. You, et al. 2021.TGF-β/Smad signalling in neurogenesis: implications for neuropsychiatric diseases. Cells 10(6):1382.
Garcia-Bueno, B., J.R. Caso, and J.C. Leza. 2008. Stress as a neuroinflammatory condition in brain: Damaging and protective mechanisms. Neuroscience and Biobehavioral Reviews 32: 1136–1151.
Sotiropoulos, M.G., and T. Chitnis. 2020. Opposing and potentially antagonistic effects of BMP and TGF-β in multiple sclerosis: The “Yin and Yang” of neuro-immune Signaling. Journal of Neuroimmunology 347: 577358.
Vogel, T., S. Ahrens, N. Büttner, et al. 2010. Transforming growth factor beta promotes neuronal cell fate of mouse cortical and hippocampal progenitors in vitro and in vivo: Identification of Nedd9 as an essential signaling component. Cerebral Cortex 20 (3): 661–671.
Wachs, F.P., B. Winner, S. Couillard-Despres, et al. 2006. Transforming growth factor-beta1 is a negative modulator of adult neurogenesis. Journal of Neuropathology & Experimental Neurology 65: 358–370.
Chao, C.C., S. Hu, W.H. Frey, et al. 1994. Transforming growth factor beta in Alzheimer’s disease. Clinical and Diagnostic Laboratory Immunology 1: 109–110.
Vawter, M.P., O. Dillon-Carter, W.W. Tourtellotte, et al. 1996. TGFbeta1 and TGFbeta2 concentrations are elevated in Parkinson’s disease in ventricular cerebrospinal fluid. Experimental Neurology 142: 313–322.
Tzavlaki, K., and A. Moustakas. 2020. TGF-β signaling. Biomolecule 10 (3): 487.
El-Sawaf, E.S., S. Saleh, D.M. Abdallah, et al. 2021. Vitamin D and rosuvastatin obliterate peripheral neuropathy in a type-2 diabetes model through modulating Notch1, Wnt-10α, TGF-β and NRF-1 crosstalk. Life Sciences 279: 119697.
ACKNOWLEDGEMENTS
We thank Dr. Yanqiang Wang for his instructive advice and useful suggestions on my thesis, and providing to help experiment design and completion in the study.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81870943).
Author information
Authors and Affiliations
Contributions
This article is mainly written by XWL and SX. JL and YZ wrote part of the manuscript and proofread the manuscript. RH helped us collect literature information and draw pictures. XLL and YW reviewed the manuscript and proposed final revisions. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All experiments were performed according to the institutional ethical guidelines on animal care and approved by the Institute Animal Care and Use Committee at the Weifang Medical University, Shandong, China.
Consent for Publication
The consent of all coauthors was collected before submission. A preprint has previously been published.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Xu, S., Liu, J. et al. Treatment with 1,25-Dihydroxyvitamin D3 Delays Choroid Plexus Infiltration and BCSFB Injury in MRL/lpr Mice Coinciding with Activation of the PPARγ/NF-κB/TNF-α Pathway and Suppression of TGF-β/Smad Signaling. Inflammation 46, 556–572 (2023). https://doi.org/10.1007/s10753-022-01755-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-022-01755-5