Abstract
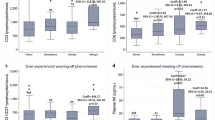
Interstitial lung disease (ILD) is a highly fatal manifestation of idiopathic inflammatory myopathies (IIMs). Th cells play important roles in the initiation of ILD. Here, we investigated the clinical significance of peripheral blood Th cells in IIMs-ILD patients. Eleven healthy controls (HC) and 53 patients diagnosed with IIMs were included, including 30 with ILD (IIMs-ILD) and 23 without ILD (IIMs-non-ILD). Circulating Th1, Th2, Th17, and Treg cells were examined by flow cytometry, and their correlation with clinical and laboratory findings was analyzed by Spearman’s correlation and logistic regression. The proportion of Th1 cells decreased and Th2 cells increased in IIMs-ILD compared with IIMs-non-ILD (median (quartile): 2.99 (1.59–5.39) vs. 6.91 (3.48–10.04), p < 0.001; 2.67 (1.79–4.67) vs. 1.62 (0.85–2.66), p = 0.006) and correlated with disease activity. The Th1-cell proportion decreased in anti-MDA5 antibody-positive patients, while the Th2 cell proportion increased in patients with nonspecific interstitial pneumonia compared with IIMs-non-ILD (2.66 (1.06–4.35) vs. 6.91 (3.48–10.04), p = 0.002; 3.09 (2.03–5.72) vs. 1.62 (0.85–2.66), p = 0.016). Univariate analysis showed that a lower Th1 proportion, higher Th2 proportion increased, lower CK level, positivity for ARS, or anti-Ro52 antibodies (OR = 0.7122; OR = 1.679; OR = 0.9993; OR = 9.188; and OR = 6.161, respectively) were associated with the occurrence of ILD in IIMs patients. Decreased Th1 cells and elevated Th2 cells in peripheral blood may be involved in the pathogenesis of ILD in IIMs patients and have different effects on different serological and imaging subtypes.




Similar content being viewed by others
DATA AVAILABILITY
The data supporting the conclusions of this article are included within the article and its additional file.
References
Lundberg, I.E., M. Fujimoto, J. Vencovsky, R. Aggarwal, M. Holmqvist, L. Christopher-Stine, A.L. Mammen, and F.W. Miller. 2021. Idiopathic inflammatory myopathies. Nature Reviews. Disease Primers 7 (1): 86. https://doi.org/10.1038/s41572-021-00321-x.
Vegosen, L.J., C.R. Weinberg, T.P. O’Hanlon, I.N. Targoff, F.W. Miller, and L.G. Rider. 2007. Seasonal birth patterns in myositis subgroups suggest an etiologic role of early environmental exposures. Arthritis and Rheumatism 56 (8): 2719–2728. https://doi.org/10.1002/art.22751.
Rothwell, S., R.G. Cooper, I.E. Lundberg, F.W. Miller, P.K. Gregersen, J. Bowes, J. Vencovsky, K. Danko, V. Limaye, A. Selva-O'Callaghan, M.G. Hanna, P.M. Machado, L.M. Pachman, A.M. Reed, L.G. Rider, J. Cobb, H. Platt, Ø. Molberg, O. Benveniste, P. Mathiesen, T. Radstake, A. Doria, J. De Bleecker, B. De Paepe, B. Maurer, W.E. Ollier, L. Padyukov, T.P. O'Hanlon, A. Lee, C.I. Amos, C. Gieger, T. Meitinger, J. Winkelmann, L.R. Wedderburn, H. Chinoy, J.A. Lamb. 2016. Myositis Genetics Consortium. Dense genotyping of immune-related loci in idiopathic inflammatory myopathies confirms HLA alleles as the strongest genetic risk factor and suggests different genetic background for major clinical subgroups. Annals of the Rheumatic Diseases 75(8):1558–66. https://doi.org/10.1136/annrheumdis-2015-20811
Barba, T., R. Fort, V. Cottin, S. Provencher, I. Durieu, S. Jardel, A. Hot, Q. Reynaud, and J.C. Lega. 2019. Treatment of idiopathic inflammatory myositis associated interstitial lung disease: A systematic review and meta-analysis. Autoimmunity Reviews 18 (2): 113–122. https://doi.org/10.1016/j.autrev.2018.07.013.
Wu, W., L. Guo, Y. Fu, K. Wang, D. Zhang, W. Xu, Z. Chen, and S. Ye. 2021. Interstitial lung disease in anti-MDA5 positive dermatomyositis. Clinical Reviews in Allergy and Immunology 60 (2): 293–304. https://doi.org/10.1007/s12016-020-08822-5.
Wells, A.U., and C.P. Denton. 2014. Interstitial lung disease in connective tissue disease–mechanisms and management. Nature Reviews Rheumatology 10 (12): 728–739. https://doi.org/10.1038/nrrheum.2014.149.
Geginat, J., M. Paroni, F. Facciotti, P. Gruarin, I. Kastirr, F. Caprioli, M. Pagani, and S. Abrignani. 2013. The CD4-centered universe of human T-cell subsets. Seminars in Immunology 25 (4): 252–262. https://doi.org/10.1016/j.smim.2013.10.012.
Zhang, M., and S. Zhang. 2020. T cells in fibrosis and fibrotic diseases. Frontiers in Immunology 11: 1142. https://doi.org/10.3389/fimmu.2020.01142.
Bohan, A., and J.B. Peter. 1975. Polymyositis and dermatomyositis (first of two parts) (J). New England Journal of Medicine 292 (7): 344–347. https://doi.org/10.1056/NEJM197502132920706.
Rider, L.G., V.P. Werth, A.M. Huber, et al. 2011. Measures of adult and juvenile dermatomyositis, polymyositis, and inclusion body myositis: Physician and Patient/Parent Global Activity, Manual Muscle Testing (MMT), Health Assessment Questionnaire (HAQ)/Childhood Health Assessment Questionnaire (C-HAQ), Childhood Myositis Assessment Scale (CMAS), Myositis Disease Activity Assessment Tool (MDAAT), Disease Activity Score (DAS), Short Form 36 (SF-36), Child Health Questionnaire (CHQ), physician global damage, Myositis Damage Index (MDI), Quantitative Muscle Testing (QMT), Myositis Functional Index-2 (FI2), Myositis Activities Profile (MAP), Inclusion Body Myositis Functional Rating Scale (IBMFRS), Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), Cutaneous Assessment Tool (CAT), Dermatomyositis Skin Severity Index (DSSI), Skindex, and Dermatology Life Quality Index (DLQI). Arthritis Care and Research (Hoboken) 63 Suppl 11(0 11):S118–57. https://doi.org/10.1002/acr.20532
Bruce, B., and J.F. Fries. 2003. The Stanford Health Assessment Questionnaire: Dimensions and practical applications(J). Health and Quality of Life Outcomes 1: 20. https://doi.org/10.1186/1477-7525-1-20.
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. 2002. ATS statement: guidelines for the six-minute walk test. American Journal of Respiratory and Critical Care Medicine 166(1):111–117. https://doi.org/10.1164/ajrccm.166.1.at1102
Travis, W.D., U. Costabel, D.M. Hansell, et al. 2013. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. American Journal of Respiratory and Critical Care Medicine 188(6):733–48. https://doi.org/10.1164/rccm.201308-1483ST
Flaherty, K.R., E.L. Thwaite, E.A. Kazerooni, B.H. Gross, G.B. Toews, T.V. Colby, W.D. Travis, J.A. Mumford, S. Murray, A. Flint, J.P. Lynch 3rd., and F.J. Martinez. 2003. Radiological versus histological diagnosis in UIP and NSIP: Survival implications. Thorax 58 (2): 143–148. https://doi.org/10.1136/thorax.58.2.143.
Romagnani, S. 2000. T-cell subsets (Th1 versus Th2). Annals of Allergy, Asthma and Immunology 85(1):9–18; quiz 18, 21. https://doi.org/10.1016/S1081-1206(10)62426-X
Wynn, T.A. 2004. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nature Reviews Immunology 4 (8): 583–594. https://doi.org/10.1038/nri1412.
Keane, M.P., J.A. Belperio, M.D. Burdick, and R.M. Strieter. 2001. IL-12 attenuates bleomycin-induced pulmonary fibrosis. American Journal of Physiology. Lung Cellular and Molecular Physiology 281 (1): L92–L97. https://doi.org/10.1152/ajplung.2001.281.1.L92.
Gono, T., H. Kaneko, Y. Kawaguchi, M. Hanaoka, S. Kataoka, M. Kuwana, K. Takagi, H. Ichida, Y. Katsumata, Y. Ota, H. Kawasumi, and H. Yamanaka. 2014. Cytokine profiles in polymyositis and dermatomyositis complicated by rapidly progressive or chronic interstitial lung disease. Rheumatology (Oxford) 53 (12): 2196–2203. https://doi.org/10.1093/rheumatology/keu258.
Abbas, A.K., E.R. Trotta, D. Simeonov, A. Marson, J.A. Bluestone. 2018. Revisiting IL-2: Biology and therapeutic prospects. Science Immunology 6;3(25):eaat1482. https://doi.org/10.1126/sciimmunol.aat1482.
Frantz, C., A. Cauvet, A. Durand, V. Gonzalez, R. Pierre, M. Do Cruzeiro, K. Bailly, M. Andrieu, C. Orvain, J. Avouac, M. Ottaviani, R. Thuillet, L. Tu, C. Guignabert, B. Lucas, C. Auffray, and Y. Allanore. 2022. IL-2-related regulatory CD4 T-cell deficiency leads to the development of lung fibrosis and vascular remodeling. Arthritis & Rhematology. https://doi.org/10.1002/art.42111.
Feng, M., H. Guo, C. Zhang, Y. Wang, Z. Liang, X. Zhao, Y. Qin, Y. Wu, G. Liu, C. Gao, and J. Luo. 2019. Absolute reduction of regulatory T cells and regulatory effect of short-term and low-dose IL-2 in polymyositis or dermatomyositis. International Immunopharmacology 77: 105912. https://doi.org/10.1016/j.intimp.2019.105912.
Zou, J., T. Li, X. Huang, S. Chen, Q. Guo, and C. Bao. 2014. Basiliximab may improve the survival rate of rapidly progressive interstitial pneumonia in patients with clinically amyopathic dermatomyositis with anti-MDA5 antibody. Annals of the Rheumatic Diseases 73 (8): 1591–1593. https://doi.org/10.1136/annrheumdis-2014-205278.
Gieseck, R.L., 3rd., M.S. Wilson, and T.A. Wynn. 2018. Type 2 immunity in tissue repair and fibrosis. Nature Reviews Immunology 18 (1): 62–76. https://doi.org/10.1038/nri.2017.90.
Shen, H., L. Xia, and J. Lu. 2013. Interleukin-4 in rheumatoid arthritis patients with interstitial lung disease: A pilot study. Indian Journal of Medical Research 138 (6): 919–921.
Maher, T.M., U. Costabel, M.K. Glassberg, Y. Kondoh, T. Ogura, M.B. Scholand, D. Kardatzke, M. Howard, J. Olsson, M. Neighbors, P. Belloni, and J.J. Swigris. 2021. Phase 2 trial to assess lebrikizumab in patients with idiopathic pulmonary fibrosis. European Respiratory Journal 57 (2): 1902442. https://doi.org/10.1183/13993003.02442-2019.
Distler, J.H.W., A.H. Györfi, M. Ramanujam, M.L. Whitfield, M. Königshoff, and R. Lafyatis. 2019. Shared and distinct mechanisms of fibrosis. Nature Reviews Rheumatology 15 (12): 705–730. https://doi.org/10.1038/s41584-019-0322-7.
Keogh, K.A., and A.H. Limper. 2005. Characterization of lymphocyte populations in nonspecific interstitial pneumonia. Respiratory Research 6 (1): 137. https://doi.org/10.1186/1465-9921-6-137.
Shi, L., J. Wang, H.X. Guo, X.L. Han, Y.P. Tang, and G.Y. Liu. 2022. Circulating Th2 cell reduction and Th1/Th2 imbalance are correlated with primary Sjogren’s syndrome-associated interstitial lung disease. Arthritis Research & Therapy 24 (1): 121. https://doi.org/10.1186/s13075-022-02811-z.
Divekar, A.A., D. Khanna, F. Abtin, P. Maranian, R. Saggar, R. Saggar, D.E. Furst, and R.R. Singh. 2011. Treatment with imatinib results in reduced IL-4-producing T cells, but increased CD4+ T cells in the broncho-alveolar lavage of patients with systemic sclerosis. Clinical Immunology 141 (3): 293–303. https://doi.org/10.1016/j.clim.2011.08.010.
Huang, H.L., W.C. Lin, C.C. Yeh, and Y.T. Sun. 2020. Serological risk factors for concomitant interstitial lung disease in patients with idiopathic inflammatory myopathy. Journal of Clinical Neuroscience 74: 32–35. https://doi.org/10.1016/j.jocn.2020.01.060.
Marie, I., S. Josse, O. Decaux, S. Dominique, E. Diot, C. Landron, P. Roblot, S. Jouneau, P.Y. Hatron, K.P. Tiev, O. Vittecoq, D. Noel, L. Mouthon, J.F. Menard, and F. Jouen. 2012. Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmunity Reviews 11 (10): 739–745. https://doi.org/10.1016/j.autrev.2012.01.006).
Ferreira, B.S.A., B.M.D. Cunha, L.A. Moreira, M.F.S.E. Fonseca, and E.B.U. Cavalcante. 2022. Comments: Myositis-specific and myositis-associated autoantibody profiles and their clinical associations in a large series of patients with polymyositis and dermatomyositis. Clinics (São Paulo, Brazil) 23 (77): 100077. https://doi.org/10.1016/j.clinsp.2022.100077.
Funding
This work was supported by the National Natural Science Foundation of China (82001728,81973540), the 1·3·5 project for Outstanding interdisciplinary project of West China Hospital, Sichuan University (ZYGD18015, ZYJC18003, ZYJC18024), the Sichuan Science and Technology Program (20YYJC3358), and the zero to one Innovative Research Program of Sichuan University (2022SCUH0020).
Author information
Authors and Affiliations
Contributions
LY, TCY, and LYB conceived and designed the study. LY and TCY guided the study. CL, LYH, ZY, WJ, WYL, LXP, and WT collected the clinical samples and assessed the disease activity. CL, LYH, LYB, ZY, WYL, WJ, and LXP performed flow cytometry and analyzed the data. All authors drafted and revised the manuscript. All authors drafted and revised the manuscript. Lu Cheng and Yanhong Li contributed equally to this work.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
The study was approved by the ethical committee of West China Hospital of Sichuan University (no. 695 in 2020) and complied with the Declaration of Helsinki. The study did not involve animal studies, and no ethical approval was needed.
Consent for Publication
All patients and controls provided written informed consent.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, L., Li, Y., Luo, Y. et al. Decreased Th1 Cells and Increased Th2 Cells in Peripheral Blood Are Associated with Idiopathic Inflammatory Myopathies Patients with Interstitial Lung Disease. Inflammation 46, 468–479 (2023). https://doi.org/10.1007/s10753-022-01747-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-022-01747-5