Abstract
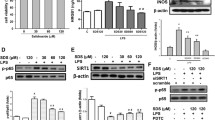
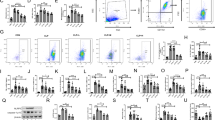
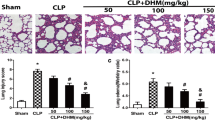
Acute lung injury (ALI) is a common lung disease characterized by severe acute inflammatory lung injury in patients with sepsis. Syringaresinol (SYR) has been reported to have anti-apoptotic and anti-inflammatory effects, but whether it could prevent pyroptosis to improve sepsis-induced ALI remains unclear. The purpose of this work was to examine the impact of SYR on sepsis-induced ALI and investigate the underlying mechanisms. The ALI model was induced by caecal ligation and puncture (CLP) in C57BL/6 mice, structural damage in the lung tissues was determined using haematoxylin and eosin (HE) staining, and the levels of related inflammatory cytokines and macrophage polarization were examined by enzyme-linked immunosorbent assays (ELISAs) and flow cytometry, respectively. The activation of the NLRP3 inflammasome and the protein levels of TLR4, NF-κB and MAPKs was measured by western blotting. The results demonstrated that SYR pretreatment significantly reduced lung tissue histological damage, inhibited the production of proinflammatory cytokines and albumin in bronchoalveolar lavage fluid (BALF), and decreased myeloperoxidase (MPO) levels, thereby alleviating lung tissue injury. Meanwhile, septic mice treated with SYR displayed a higher survival rate and lower percentage of M1 macrophages in the BALF and spleen than septic mice. In addition, lung tissues from the CLP + SYR group exhibited downregulated protein expression of NLRP3, ASC, GSDMD caspase-1 p20 and TLR4, along with decreased phosphorylated levels of NF-κB, ERK, JNK and P38, indicating that SYR administration effectively prevented CLP-induced pyroptosis in the lung. SYR also suppressed LPS-induced pyroptosis in RAW 264.7 cells by inhibiting the activation of the NLRP3 inflammasome, which was abolished by an oestrogen receptor-β (ERβ) antagonist (PHTPP). In conclusion, SYR exerted protective effects on CLP-induced ALI via the oestrogen receptor-β signalling pathway.







Similar content being viewed by others
Availability of Data and Material
All the data generated or analysed during this study are included in this published article.
Code Availability
Not applicable.
References
Ferguson, N.D., D.J. Cook, G.H. Guyatt, S. Mehta, L. Hand, and P. Austin, et al. 2013. High-frequency oscillation in early acute respiratory distress syndrome. New England Journal of Medicine 368 (9): 795–805.
Li, X.Y., J. Wang, H.S. Wu, P.P. Guo, C.Y. Wang, and Y.L. Wang, et al. 2018. Peripheral blood miR-140 may be a biomarker for acute lung injury by targeting Toll-like receptor 4 (TLR4). Experimental and Therapeutic Medicine 16 (4): 3632–3638.
Bellani, G., J.G. Laffey, T. Pham, E. Fan, L. Brochard, and A. Esteban, et al. 2016. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. Journal of the American Medical Association 315 (8): 788–800.
Mangia, C.M.F., N. Kissoon, J.A. Carcillo, et al. 2009. Sepsis and septic shock: A global overview. Journal of Pediatric Infectious Diseases 04 (02): 071–076.
Jiang, Z.L., A.L. Chen, L. Hu, L. Qiu, and L. Zhu. 2020. Calreticulin blockade attenuates murine acute lung injury by inducing polarization of M2 subtype macrophages. Frontier Immunology 30 (11): 11. https://doi.org/10.3389/fimmu.2020.00011.
Gao, W., Y. Wang, Y. Xiong, L. Sun, L. Wang, K. Wang, et al. 2019. Size-dependent anti-inflammatory activity of a peptide-gold nano-particle hybrid in vitro and in a mouse model of acute lung injury. Acta Biomaterialia 85: 203–217.
Li, D., W. Ren, Z. Jiang, and L. Zhu. 2018. Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Molecular Medicine Reports 18 (5): 4399–4409. https://doi.org/10.3892/mmr.2018.9427.
Jorgensen, I., and E.A. Miao. 2015. Pyroptotic cell death defends against intracellular pathogens. Immunological Reviews 265 (1): 130–142.
Ying, Y.G., Y. Mao, and M. Yao. 2019. NLRP3 Inflammasome activation by microRNA-495 promoter methylation may contribute to the progression of acute lung injury. Molecular Therapy-Nucleic Acids Dec 6 801–814.
Lee, E.H., J.H. Shin, S.S. Kim, H. Lee, S.R. Yang, and S.R. Seo. 2019. Laurus nobilis leaf extract controls inflammation by suppressing NLRP3 inflammasome activation. Journal of Cellular Physiology 234 (5): 6854–6864.
Yang, H.H., J.X. Duan, S.K. Liu, J.B. Xiong, X.X. Guan, and W.J. Zhon, et al. 2020. A COX-2/sEH dual inhibitor PTUPB alleviates lipopolysaccharide-induced acute lung injury in mice by inhibiting NLRP3 inflammasome activation. Theranostics 10 (11): 4749–4761.
Lamkanfi, M., and V.M. Dixit. 2014. Mechanisms and functions of inflammasomes. Cell 157: 1013–1022.
Hooftman, Alexander, Stefano Angiari, Svenja Hester, Sarah E. Corcoran, and Marah C. Runtsch, et al. 2020. The immunomodulatory metabolite itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metabolism 32 (3): 468–478.
Shi, J., Y. Zhao, K. Wang, X. Shi, Y. Wang, H. Huang, Y. Zhuang, T. Cai, and F. Wang. 2015. Shao F Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526: 660–665.
Jo, E.K., J.K. Kim, D.M. Shin, and C. Sasakawa. 2016. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 13 (2): 148–59.
Shan, Q., G.H. Zheng, X.R. Han, X. Wen, S. Wang, and M.Q. Li. 2018. Troxerutin protects kidney tissue against BDE-47-induced inflammatory damage through CXCR4-TXNIP/NLRP3 signaling. Oxidative Medicine and Cellular Longevity, 9865495.
Zhong, W.J., H.H. Yang, X.X. Guan, J.B. Xiong, C.C. Sun, C.Y. Zhang, et al. 2019. Inhibition of glycolysis alleviates lipopolysaccharide-induced acute lung injury in a mouse model. Journal of Cellular Physiology 234 (4): 4641–4654.
He, X.Y., Z. Qian, E. Li, K. Fan, Y. Li, L. Wu, T.R. Billiar, M.A. Wilson, X. Shi, and J. Fan. TLR4-upregulated IL-1β and IL-1RI promote alveolar macrophage pyroptosis and lung inflammation through an autocrine mechanism. Science Report 6 (1): 1–11.
Dong, N., X. Xu, C. Xue, C. Wang, X. Li, C. Bi, and A. Shan. 2019. Ethyl pyruvate inhibits LPS induced IPEC-J2 inflammation and apoptosis through p38 and ER K1/2 pathways. Cell Cycle 18: 2614–2628.
Zhang, Z.Q., Q. Nian, C. Chen, A.Q. Cui, Y.Z. Han, and J.Y. Zhan. 2020. Klotho. Alleviates lung injury caused by paraquat via suppressing ROS/P38 MAPK-regulated inflammatory responses and apoptosis. Oxidative Medicine and Cellular Longevity 13 (2020): 1854206.
Altemimi, A., N. Lakhssassi, N. Baharlouei, D.G. Watson, and D.A. Lightfoot. 2017. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 6 (4): 42.
Habib, M., M. Trajkovic, and M.W. Fraaije. 2018. The biocatalytic synthesis of syringaresinol from 2,6-dimethoxy-4-allylphenol in One-Pot using a tailored oxidase/peroxidase system. ACS Catalysis 8 (6): 5549–5552.
Kim, J., T. Toda, K. Watanabe, S. Shibuya, Y. Ozawa, N. Izuo, and Cho, et al. 2019. Syringaresinol reverses age-related skin atrophy by suppressing FoxO3a-mediated matrix metalloproteinase -2 activation in copper/zinc superoxide dismutase-deficient mice. The Journal of Investigative Dermatology 139: 648–655.
Oh, J.H., Y.H. Joo, F. Karadeniz, J. Ko, C.S. Kong. 2020. Syringaresinol inhibits UVA-induced MMP-1 expression by suppression of MAPK/AP-1 signaling in HaCaT keratinocytes and human dermal fibroblasts. International Journal of Molecular Science 21(11): 3981.
Bajpai, V.K., M.B. Alam, K.T. Quan, M.K. Ju, R. Majumder, and S. Shukla, et al. 2018. Attenuation of inflammatory responses by (+)-syringaresinol via MAP-kinase-mediated suppression of NF-kappaB signaling in vitro and in vivo. Science Reports 8 (1): 9216.
Cho, Y.S., W.S. Song, S.H. Yoon, K.Y. Park, and M.H. Kim. 2018. Syringaresinol suppresses excitatory synaptic transmission and picrotoxin-induced epileptic activity in the hippocampus through presynaptic mechanisms Neuropharmacology. 131: 68–82.
Li, G.R., L. Yang, L.F. Feng, J.Yang, Y.F. Li, J. An, D.H. Li, et al. 2020. Syringaresinol protects against type 1 diabetic cardiomyopathy by alleviating inflammation responses, cardiac fibrosis and oxidative stress. Molecular Nutrition & Food Research 30: e2000231
Zhang, Z., L. Yang, B. Wang, L. Zhang, Q. Zhang, D. Li, S. Zhang, H. Gao, and X. Wang. 2017. Protective role of liriodendrin in mice with dextran sulphate sodium-induced ulcerative colitis. International Immunopharmacology 52: 203–210.
Zhuo, Y., S. Zhang, C. Li, L. Yang, H. Gao, and X. Wang. 2018. Resolvin D1 promotes SIRT1 expression to counteract the activation of STAT3 and NF-κB in mice with septic associated lung injury. Inflammation 41 (5): 1762–1771.
Hou, L.C., M.Z. Qin, L.N. Zheng, Y. Lu, Q. Wang, D.R. Peng, X.P. Yu, Y.C. Xin, G.L. Ji, and L.Z. Xiong. 2009. Severity of sepsis is correlated with the elevation of serum high-mobility group box 1 in rats. Chinese Medical Journal 122 (4): 449–454.
Taratummarat, S., N. Sangphech, C.T.B. Vu, T. Palaga, T. Ondee, S. Surawut, A. Sereemaspun, P. Ritprajak, and A. Leelahavanichkul. 2018. Gold nanoparticles attenuates bacterial sepsis in cecal ligation and puncture mouse model through the induction of M2 macrophage polarization. BMC Microbiology 18 (1): 85.
Zhang, J., Y. Wang. Luo, X. Zhu, J. Li, Q. Feng, J., et al. 2019. Global transcriptional regulation of STAT3- and MYC-mediated sepsis-induced ARDS. Therapeutic Advances in Respiratory Disease 13: 1753466619879840.
Gao, W., Y. Wang, Y. Xiong, L. Sun, L. Wang, ang K. Wang, et al. 2019. Size-dependent anti-inflammatory activity of a peptide-gold nanoparticle hybrid in vitro and in a mouse model of acute lung injury. Acta Biomaterilia 85: 203–217.
Han, S., and R.K. Mallampalli. 2015. The acute respiratory distress syndrome: from mechanism to translation. Journal of Immunology 194 (3): 855–860.
Xia, W.F., Z. Pan, H.M. Zhang, Q.S. Zhou, and Y. Liu. 2020. Inhibition of ERR α aggravates sepsis-induced acute lung injury in rats via provoking inflammation and oxidative stress. Oxidative Medicine Cellular Longevity 2020: 2048632. https://doi.org/10.1155/2020/2048632.
Liu, M.Y., Y.L. Chen, S.Y. Wang, H.P. Zhou, D.Feng, and J. Wei, et al. 2020. α-Ketoglutarate modulates macrophage polarization through regulation of PPARγ transcription and mTORC1/p70S6K pathway to ameliorate ALI/ARDS. Shock. 53 (1): 103–113.
Yang, X.L., Y.B. Yin, X.X. Yan, Z.B. Yu, Y. Liu, and J. Cao. 2019. Flagellin attenuates experimental sepsis in a macrophage-dependent manner. Critical Care Apr 3 23 (1): 106.
Wang, N., H. Liang, and K. Zen. 2014. Molecular mechanisms that influence the macrophage m1–m2 polarization balance. Frontiers in Immunology 5: 614.
Ying, Y., Y. Mao, and M. Yao. 2019. NLRP3 inflammasome activation by microRNA-495 promoter methylation may contribute to the progression of acute lung injury. Molecular Therapy Nucleic Acids 18: 801–814. https://doi.org/10.1016/j.omtn.2019.08.028.
Peng, F., W. Chang, and Q Sun, et al. 2020. HGF alleviates septic endothelial injury by inhibiting pyroptosis via the mTOR signalling pathway. Respiratory Research 21 (1): 215. Published 2020 Aug 14. https://doi.org/10.1186/s12931-020-01480-3.
Youguo, Ying, Yong Mao, and Min Yao. 2019. NLRP3 inflammasome activation by microRNA-495 promoter methylation may contribute to the progression of acute lung injury. Molecular Therapy-Nucleic Acids Dec 6 18:801–814. https://doi.org/10.1016/j.omtn.2019.08.028.
Li, Dandan, Weiying Ren, and Zhilong Jiang. 2018. Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Molecular Medicine Reports 18: 4399–4409.
Bauernfeind, F.G., G. Horvath, A. Stutz, E.S. Alnemri, K. MacDonald, D. Speert, T. Fernandes-Alnemri, J. Wu, et al. 2009. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. The Journal of Immunology 183: 787–791.
Song, N., Z.S. Liu, W. Xue, Z.F. Bai, Q.Y. Wang, J. Dai, et al. 2017. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Molecular Cell 68 (1): 185–197.
Heinonen, S., T. Nurmi, K. Liukkonen, K. Poutanen, K. Wähälä, T. Deyama, S. Nishibe, and H. Adlercreutz. 2001. In vitro metabolism of plant lignans: New precursors of mammalian lignans enterolactone andenterodiol. Journal of Agriculture and Food Chemistry 49 (7): 3178–3186.
Abdullaha, M., M. Ali, D. Kour, A. Kumar, and S.B. Bharate. 2020. Discovery of benzo [cd] indol-2-one and benzylidene-thiazolidine-2, 4-dione as new classes of NLRP3 inflammasome inhibitors via ER-β structure based virtual screening. Bioorganic Chemistry 95: 103500.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 81971858), Tianjin Natural Science Foundation Youth Fund (18JCQNJC13400; 19JCZDJC36200), the Science Foundation of Shenzhen Municipal Health Bureau (201902) and the Scientific Research Project of Integrated Traditional Chinese and Western Medicine of Tianjin Health Commission (201938).
Author information
Authors and Affiliations
Contributions
Jiarui Li and Ximo Wang designed this study. Yuzhen Zhuo and Lei Yang performed the experiment. Dihua Li analysed and interpreted the data, Lanqiu Zhang wrote the manuscript. Qi Zhang, Shukun Zhang, Caixia Li, Lihua Cui and Jian Hao participated in the experimental operation.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
All the authors agree to submit the final version of manuscript for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhuo, Y., Yang, L., Li, D. et al. Syringaresinol Resisted Sepsis-Induced Acute Lung Injury by Suppressing Pyroptosis Via the Oestrogen Receptor-β Signalling Pathway. Inflammation 45, 824–837 (2022). https://doi.org/10.1007/s10753-021-01587-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01587-9