Abstract—
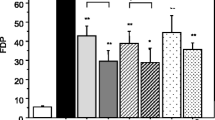
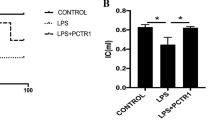
Endothelial glycocalyx degradation is thought to facilitate the development of sepsis. Histone is a significant mediator in sepsis. Unfractionated heparin (UFH) possessed beneficial effects on sepsis. Thereby, this study aims to figure out whether histone can disrupt glycocalyx and to investigate the protective effect and mechanism of UFH. Male mice (C57BL/6, 8–10 weeks old, weighing 20–25 g) were randomly divided into five groups including control group, histone group, histone + UFH group, histone + heparinase (HPA) inhibitor group, and histone + UFH + HPA inhibitor group. The mice were treated with histone (50 mg/kg) via tail vein immediately after HPA (20 mg/kg) injection. UFH (400 U/kg) was injected 1h after histone administration. The other groups were injected with equal volume of sterile saline accordingly. UFH alleviated histone-induced lung injury and pulmonary edema. UFH inhibited histone-induced lung coagulation activation and inflammatory response. UFH treatment markedly inhibited pulmonary glycocalyx degradation by reducing the histone-induced decrease in the levels of lung syndecan-1 mRNA and protein. UFH downregulated histone-induced expression of HPA mRNA and protein, and thus alleviated glycocalyx degradation. UFH protects against histone-induced pulmonary glycocalyx injury partly by heparinase pathway.






Similar content being viewed by others
References
Singer, M., C.S. Deutschman, C.W. Seymour, M. Shankar-Hari, D. Annane, M. Bauer, R. Bellomo, et al. 2016. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315(8): 801-810. https://doi.org/10.1001/jama.2016.0287.
Prescott, H.C., and D.C. Angus. 2018. Enhancing Recovery From Sepsis: A Review. JAMA 319(1): 62-75. https://doi.org/10.1001/jama.2017.17687.
Allam, R., S.V.R. Kumar, M.N. Darisipudi, and H. Anders. 2014. Extracellular histones in tissue injury and inflammation. Journal of Molecular Medicine 92(5): 465-472. https://doi.org/10.1007/s00109-014-1148-z.
Xu, J., X.M. Zhang, M. Monestier, N.L. Esmon, and C.T. Esmon. 2011. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. Journal of Immunology 187(5): 2626–2631. https://doi.org/10.4049/jimmunol.1003930.
Ammollo, C.T., F. Semeraro, J. Xu, N.L. Esmon, and C.T. Esmon. 2011. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. Journal of Thrombosis and Haemostasis 9(9): 1795–1803. https://doi.org/10.1111/j.1538-7836.2011.04422.x.
Xu, J., X. Zhang, R. Pelayo, M. Monestier, C.T. Ammollo, F. Semeraro, F.B. Taylor, N.L. Esmon, F. Lupu, and C.T. Esmon. 2009. Extracellular histones are major mediators of death in sepsis. Nature Medicine 15(11): 1318–1321. https://doi.org/10.1038/nm.2053.
Abrams, S.T., N. Zhang, J. Manson, T.T. Liu, C. Dart, F. Baluwa, S.S. Wang, et al. 2013. Circulating histones are mediators of trauma-associated lung injury. American journal of respiratory and critical care medicine. 187(2): 160–169. https://doi.org/10.1164/rccm.201206-1037OC.
Reitsma, S., D.W. Slaaf, H. Vink, M.A.M.J. van Zandvoort, and G.A.M oude Egbrink. 2007. The endothelial glycocalyx: Composition functions and visualization. Pflugers Archiv: European journal of physiology 454(3): 345–359. https://doi.org/10.1007/s00424-007-0212-8.
Wang, W. 2007. Change in properties of the glycocalyx affects the shear rate and stress distribution on endothelial cells. Journal of Biomechanical Engineering 129(3): 324–329. https://doi.org/10.1115/1.2720909.
Becker, B.F., M. Jacob, S. Leipert, A.H.J. Salmon, and D. Chappell. 2015. Degradation of the endothelial glycocalyx in clinical settings: Searching for the sheddases. British Journal of Clinical Pharmacology 80(3): 389–402. https://doi.org/10.1111/bcp.12629.
Salmon, A.H.J., and S.C. Satchell. 2012. Endothelial glycocalyx dysfunction in disease: Albuminuria and increased microvascular permeability. The Journal of Pathology 226(4): 562–574. https://doi.org/10.1002/path.3964.
Yang, Y., V. Macleod, H.Q. Miao, A. Theus, F.H. Zhan, J.D. Shaughnessy, J. Sawyer, et al. 2007. Heparanase enhances syndecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis. The Journal of biological chemistry 282(18): 13326-13333. https://doi.org/10.1074/jbc.M611259200.
Schmidt, E.P., Y. Yang, W.J. Janssen, A. Gandjeva, M.J. Perez, L. Barthel, R.L. Zemans, et al. 2012. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nature medicine 18(8): 1217–1223. https://doi.org/10.1038/nm.2843.
Sallisalmi, M., J. Tenhunen, R. Yang, N. Oksala, and V. Pettilä. 2012. Vascular adhesion protein-1 and syndecan-1 in septic shock. Acta anaesthesiologica Scandinavica 56(3): 316-322. https://doi.org/10.1111/j.1399-6576.2011.02578.x.
Piotti, A., D. Novelli, J.M.T. Anna Meessen, D. Ferlicca, S. Coppolecchia, A. Marino, and G. Salati, et al. 2021. Endothelial damage in septic shock patients as evidenced by circulating syndecan-1 sphingosine-1-phosphate and soluble VE-cadherin: a substudy of ALBIOS. Critical care (London, England) 25(1): 113. https://doi.org/10.1186/s13054-021-03545-1.
Zhang, X.J., and X. Li. 2021. The Role of Histones and Heparin in Sepsis: A Review. Journal of intensive care medicine 885066621992320. https://doi.org/10.1177/0885066621992320.
Sun, Y.N., H. Zhang, X. An, and X.C. Ma. 2015. Effect of unfractionated heparin on endothelial glycocalyx in a septic shock model. Acta Anaesthesiologica Scandinavica 59(2): 160–169. https://doi.org/10.1111/aas.12418.
Li, X., and X. Ma. 2017. The role of heparin in sepsis: Much more than just an anticoagulant. British Journal of Haematology 179(3): 389–398. https://doi.org/10.1111/bjh.14885.
Li, L., S.H. Yu, S.F. Fu, X.C. Ma, and X. Li. 2020. Unfractionated heparin inhibits histone-mediated coagulation activation and thrombosis in mice. Thrombosis research 193: 122-129. https://doi.org/10.1016/j.thromres.2020.06.007.
Shimada, K., S. Anai, T. Fujii, N. Tanaka, K. Fujimoto, and N. Konishi. 2013. Syndecan-1 (CD138) contributes to prostate cancer progression by stabilizing tumour-initiating cells. The Journal of pathology 231(4): 495-504. https://doi.org/10.1002/path.4271.
Ding, R.Y., D.M. Zhao, R.X. Guo, Z.D. Zhang, and X.C. Ma. 2011. Treatment with unfractionated heparin attenuates coagulation and inflammation in endotoxemic mice. Thrombosis Research 128(6): e160–e165. https://doi.org/10.1016/j.thromres.2011.07.044.
Alhamdi, Y., M. Zi, S.T. Abrams, T. Liu, Su. Dunhao, I. Welters, T. Dutt, E.J. Cartwright, G. Wang, and C.H. Toh. 2016. Circulating Histone Concentrations Differentially Affect the Predominance of Left or Right Ventricular Dysfunction in Critical Illness. Critical care medicine 44(5): e278-e288. https://doi.org/10.1097/CCM.0000000000001413.
Song, W.Y., X.H. Jiang, Y. Ding, Y. Wang, M.X. Zhou, Y. Xia, C.Y. Zhang CY, et al. 2020. Inhibition of heparanase protects against pancreatic beta cell death in streptozotocin-induced diabetic mice via reducing intra-islet inflammatory cell infiltration. British Journal of Pharmacology 177(19): 4433-4447. https://doi.org/10.1111/bph.15183.
Chappell, D., M. Jacob, O. Paul, M. Rehm, U. Welsch, M. Stoeckelhuber, P. Conzen, and B.F. Becker. 2009. The glycocalyx of the human umbilical vein endothelial cell: An impressive structure ex vivo but not in culture. Circulation research 104(11): 1313-1317. Circulation research. https://doi.org/10.1161/CIRCRESAHA.108.187831.
Matute-Bello, G., G. Downey, B.B. Moore, S.D. Groshong, M.A. Matthay, A.S. Slutsky, and W.M. Kuebler. 2011. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. American journal of respiratory cell and molecular biology 44(5): 725-738. https://doi.org/10.1165/rcmb.2009-0210ST.
Yu, Y., A.N. Böing, C.M. Hau, N.Hajji, W. Ruf, A. Sturk, and R. Nieuwland. 2018. Tissue Factor Coagulant Activity is Regulated by the Plasma Membrane Microenvironment. Thrombosis and haemostasis 118(6): 990-1000. https://doi.org/10.1055/s-0038-1642031.
Hakimi, S., S.S. Caroline, C. Hesse, and A. Jeppsson. 2019. Effects of fibrinogen and platelet transfusion on coagulation and platelet function in bleeding cardiac surgery patients. Acta anaesthesiologica Scandinavica 63(4): 475-482. https://doi.org/10.1111/aas.13295.
Xiong, S., Z. Hong, L.S. Huang, Y. Tsukasaki, S. Nepal, A. Di, M. Zhong, et al. 2020. IL-1β suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury. The Journal of clinical investigation 130(7): 3684-3698. https://doi.org/10.1172/JCI136908.
Kim, H.B., S. Soh, Y.L. Kwak, J.C. Bae, S.H. Kang, and J.W. Song. 2020. High Preoperative Serum Syndecan-1 a Marker of Endothelial Glycocalyx Degradation, and Severe Acute Kidney Injury after Valvular Heart Surgery. Journal of clinical medicine 9(6): 1803. https://doi.org/10.3390/jcm9061803.
Xu, Z.H., Y.B. Huang, P. Mao, J.R. Zhang, and Y.M. Li. 2015. Sepsis and ARDS: The Dark Side of Histones. Mediators of inflammation 2015:205054. https://doi.org/10.1155/2015/205054.
Grailer, J.J., B.A. Canning, M. Kalbitz, M.D. Haggadone, R. M. Dhond, A.V. Andjelkovic, F.S. Zetoune, and P.A. Ward. 2014. Critical role for the NLRP3 inflammasome during acute lung injury. Journal of immunology 192(12): 5974-5983. https://doi.org/10.4049/jimmunol.1400368.
Fuchs, T.A., A.A. Bhandari, and D.D. Wagner. 2011. Histones induce rapid and profound thrombocytopenia in mice. Blood 118(13): 3708-3714. https://doi.org/10.1182/blood-2011-01-332676.
Vassiliou, A.G., A. Kotanidou, I. Dimopoulou, and S.E. Orfanos. 2020. Endothelial Damage in Acute Respiratory Distress Syndrome. International Journal of Molecular Sciences 21(22): 8793. https://doi.org/10.3390/ijms21228793.
Joffre, J., J. Hellman, C. Ince, and H. Ait-Oufella. 2020. Endothelial Responses in Sepsis. American journal of respiratory and critical care medicine 202(3): 361-370. https://doi.org/10.1164/rccm.201910-1911TR.
Pries, A.R., and W.M. Kuebler. 2006. Normal endothelium. Handbook of experimental pharmacology (176 Pt 1):1-40. https://doi.org/10.1007/3-540-32967-6_1.
Keyloun, J.W., T.D. Le, A.E. Pusateri, R.L. Ball, B.C. Carney, T. Orfeo, K.E. Brummel-Ziedins, et al. 2020. Circulating Syndecan-1 and Tissue Factor Pathway Inhibitor Biomarkers of Endothelial Dysfunction Predict Mortality in Burn Patients. Shock 56(2): 237-244. https://doi.org/10.1097/SHK.0000000000001709.
Holzmann, M.S., M.S. Winkler, M.S. Strunden, J.R. Izbicki, G. Schoen, . Greiwe, H.O. Pinnschmidt, et al. 2018. Syndecan-1 as a biomarker for sepsis survival after major abdominal surgery. Biomarkers in medicine 12(2): 119-127. https://doi.org/10.2217/bmm-2017-0231.
Van den Berg, B.M., M. Nieuwdorp, E.S.G. Stroes, and H. Vink. 2006. Glycocalyx and endothelial (dys) function: from mice to men. Pharmacological Report 58 Suppl:75-80.
Lipowsky, H.H. 2012. The endothelial glycocalyx as a barrier to leukocyte adhesion and its mediation by extracellular proteases. Annals of biomedical engineering 40(4): 840-848. https://doi.org/10.1007/s10439-011-0427-x.
Fux, L., N. Ilan, R.D. Sanderson, and I. Vlodavsky. 2009. Heparanase: Busy at the cell surface. Trends in biochemical sciences 34(10): 511-519. https://doi.org/10.1016/j.tibs.2009.06.005.
Purushothaman, A., L. Chen, Y. Yang, and R.D. Sanderson. 2008. Heparanase stimulation of protease expression implicates it as a master regulator of the aggressive tumor phenotype in myeloma. The Journal of biological chemistry 283(47): 32628-32636. https://doi.org/10.1074/jbc.M806266200.
Mayfosh, A.J., N. Baschuk, and M.D. Hulett. 2019. Leukocyte Heparanase: A Double-Edged Sword in Tumor Progression. Frontiers in oncology 9: 331. https://doi.org/10.3389/fonc.2019.00331.
Szatmári, T., R. Ötvös, A. Hjerpe, and K. Dobra. 2015. Syndecan-1 in Cancer: Implications for Cell Signaling Differentiation and Prognostication. Disease markers 2015:796052. https://doi.org/10.1155/2015/796052.
Goldberg, R., A. Meirovitz, N. Hirshoren, R.Bulvik, A. Binder, A.M. Rubinstein, and M. Elkin. 2013. Versatile role of heparanase in inflammation. Matrix biology: Journal of the International Society for Matrix Biology 32(5): 234-240. https://doi.org/10.1016/j.matbio.2013.02.008.
Massena, S., G. Christoffersson, E. Hjertström, E. Zcharia, I. Vlodavsky, N. Ausmees, C. Rolny, J.P. Li, and M. Phillipson. 2010. A chemotactic gradient sequestered on endothelial heparan sulfate induces directional intraluminal crawling of neutrophils. Blood 116(11): 1924-1931. https://doi.org/10.1182/blood-2010-01-266072.
Zhao, C., Z.D. Zhang, X.J. Zhang, X. Li, R. Zhu, and X.C. Ma. 2009. Evaluation of clinical effects on low-dose heparin therapy for sepsis. Zhonghua nei ke za zhi [Chinese journal of internal medicine] 48: 566–569.
Spiess, B.D. 2017. Heparin: Effects upon the Glycocalyx and Endothelial Cells. The Journal of extra-corporeal Technology 49(3): 192-197.
Wildhagen, K.C., P. García de Frutos, C.P. Reutelingsperger, R. Schrijver, C. Aresté, A. Ortega-Gómez, N.M. Deckers, H. Coenraad Hemker, O. Soehnlein, and G.A. Nicolaes. 2014. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood 123(7): 1098-1101. https://doi.org/10.1182/blood-2013-07-514984.
Freeman, C.G., C.R. Parish, K.J. Knox, J.L. Blackmore, S.A. Lobov, D.W. King, T.J. Senden, and R.W. Stephens. 2013. The accumulation of circulating histones on heparan sulphate in the capillary glycocalyx of the lungs. Biomaterials 34(22): 5670-5676. https://doi.org/10.1016/j.biomaterials.2013.03.091.
Li, X., X. Li, Z. Zheng, Y.N. Liu, and X.C. Ma. 2014. Unfractionated heparin suppresses lipopolysaccharide-induced monocyte chemoattractant protein-1 expression in human microvascular endothelial cells by blocking Krüppel-like factor 5 and nuclear factor-κB pathway. Immunobiology 219(1): 778–785. https://doi.org/10.1016/j.imbio.2014.06.005.
Zhao, D.M., R.Y. Ding, Y.N. Liu, X.H. Yin, Z.D. Zhang, and X.C. Ma. 2017. Unfractionated heparin protects the protein C system against lipopolysaccharide-induced damage in vivo and in in vitro. Experimental and therapeutic medicine 14(6): 5515-5522. https://doi.org/10.3892/etm.2017.5236.
Iba, T., N. Hashiguchi, I. Nagaoka, Y. Tabe, K. Kadota, and K. Sato. 2015. Heparins attenuated histone-mediated cytotoxicity in vitro and improved the survival in a rat model of histone-induced organ dysfunction. Intensive care medicine experimental 3(1): 36. https://doi.org/10.1186/s40635-015-0072-z.
Acknowledgements
We acknowledge all staff who helped perform this study.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81671936).
Author information
Authors and Affiliations
Contributions
Conceptualization: Sifeng Fu; methodology: Sihan Yu; formal analysis and investigation: Yilin Zhao; writing — original draft preparation: Sifeng Fu; writing — review and editing: Xu Li; funding acquisition: Xu Li; resources: Xiaochun Ma; Supervision: Xiaochun Ma.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, S., Yu, S., Zhao, Y. et al. Unfractionated Heparin Attenuated Histone-Induced Pulmonary Syndecan-1 Degradation in Mice: a Preliminary Study on the Roles of Heparinase Pathway. Inflammation 45, 712–724 (2022). https://doi.org/10.1007/s10753-021-01578-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01578-w