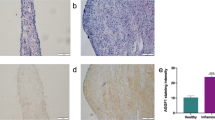
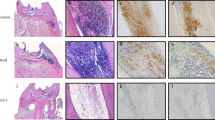
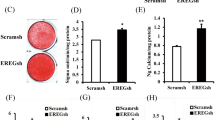
Abstract
Pulpitis is a complicated chronic inflammatory process which can be in a dynamic balance between damage and repair. The extracellular matrix plays an important regulatory role in wound healing and tissue repair. The aim of this study was to explore the role of the epigenetic mark, enhancer of zeste homolog 2 (EZH2) on the degradation of extracellular matrix during pulpitis. Quantitative polymerase chain reaction was used to assess the expression of matrix metalloproteinases (MMPs) and type I collagen in human dental pulp cells (HDPCs) upon EZH2 and EI1 (EZH2 inhibitor) stimulation. The mechanism of EZH2 affecting extracellular matrix was explored through quantitative polymerase chain reaction and Western blot. A rat model of dental pulp inflammation was established, and the expression of type I collagen in dental pulp under EZH2 stimulation was detected by immunohistochemical staining. EZH2 upregulated the expression of MMP-1, MMP-3, MMP-8, and MMP-10 and decreased the production of type I collagen in HDPCs, while EI1 had the opposite effect. EZH2 activated the nuclear factor-kappa B (NF-κB) and p38 signaling pathways in HDPCs, the inhibition of which reversed the induction of MMPs and the suppression of type I collagen. EZH2 can downregulate the type I collagen levels in an experimental model of dental pulpitis in rats. EZH2 promotes extracellular matrix degradation via nuclear factor-κB (NF-κB) and P38 signaling pathways in pulpitis. EZH2 can decrease the type I collagen levels in vivo and in vitro.





Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CK:
-
Cytokeratins
- ECM:
-
Extracellular matrix
- HDPC:
-
Human dental pulp cell
- JMJD3:
-
Jumonji domain protein 3
- MMPs:
-
Matrix metalloproteinases
- MAPK:
-
Mitogen-activated protein kinase
- NF-κB:
-
Nuclear factor-κB
- q-PCR:
-
Quantitative polymerase chain reaction
- EZH2:
-
Zeste homolog 2
References
van Amerongen, J.P., I.G. Lemmens, and G.J. Tonino. 1983. The concentration, extractability and characterization of collagen in human dental pulp. Archives of Oral Biology 28: 339–345.
Haug, S.R., and M.C. Marthinussen. 2019. Acute dental pain and salivary biomarkers for stress and inflammation in patients with pulpal or periapical inflammation. Journal of Oral & Facial Pain and Headache 33: 227–233.
Hahn, C.L., and F.R. Liewehr. 2007. Update on the adaptive immune responses of the dental pulp. Journal of Endodontia 33: 773–781.
Staquet, M.J., S.H. Durand, E. Colomb, A. Romeas, C. Vincent, F. Bleicher, et al. 2008. Different roles of odontoblasts and fibroblasts in immunity. Journal of Dental Research 87: 256–261.
Frangogiannis, N.G. 2017. The extracellular matrix in myocardial injury, repair, and remodeling. The Journal of Clinical Investigation 127: 1600–1612.
Jain, A., and R. Bahuguna. 2015. Role of matrix metalloproteinases in dental caries, pulp and periapical inflammation: an overview. Journal of Oral Biology and Craniofacial Research 5: 212–218.
Shin, S.J., J.I. Lee, S.H. Baek, and S.S. Lim. 2002. Tissue levels of matrix metalloproteinases in pulps and periapical lesions. Journal of Endodontia 28: 313–315.
Birkedal-Hansen, H. 1993. Role of matrix metalloproteinases in human periodontal diseases. Journal of Periodontology 64: 474–484.
Labrie, M., and Y. St-Pierre. 2013. Epigenetic regulation of mmp-9 gene expression. Cellular and Molecular Life Sciences 70: 3109–3124.
Moores, R.C., S. Brilha, F. Schutgens, P.T. Elkington, and J.S. Friedland. 2017. Epigenetic regulation of matrix metalloproteinase-1 and -3 expression in mycobacterium tuberculosis infection. Frontiers in Immunology 8: 602.
Hui, T., A. Peng, Y. Zhao, C. Wang, B. Gao, P. Zhang, et al. 2014. EZH2, a potential regulator of dental pulp inflammation and regeneration. Journal of Endodontia 40: 1132–1138.
De Santa, F., M.G. Totaro, E. Prosperini, S. Notarbartolo, G. Testa, and G. Natoli. 2007. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell 130: 1083–1094.
Thompson, W.L., and L.J. Van Eldik. 2009. Inflammatory cytokines stimulate the chemokines CCL2/MCP-1 and CCL7/MCP-3 through NFkB and MAPK dependent pathways in rat astrocytes [corrected]. Brain Research 1287: 47–57.
Morsczeck, C., and T.E. Reichert. 2018. Dental stem cells in tooth regeneration and repair in the future. Expert Opinion on Biological Therapy 18: 187–196.
Zhao, Y., C.L. Wang, R.M. Li, T.Q. Hui, Y.Y. Su, Q. Yuan, X.D. Zhou, and L. Ye. 2014. Wnt5a promotes inflammatory responses via nuclear factor kappaB (NF-kappaB) and mitogen-activated protein kinase (MAPK) pathways in human dental pulp cells. The Journal of Biological Chemistry 289: 21028–21039.
Bei, Y., H. Tianqian, Y. Fanyuan, L. Haiyun, L. Xueyang, Y. Jing, et al. 2017. ASH1L suppresses matrix metalloproteinase through mitogen-activated protein kinase signaling pathway in pulpitis. Journal of Endodontics 43: 306–314.e2.
Liu, T.P., H.L. Lo, L.S. Wei, H.H. Hsiao, and P.M. Yang. 2015. S-Adenosyl-L-methionine-competitive inhibitors of the histone methyltransferase EZH2 induce autophagy and enhance drug sensitivity in cancer cells. Anti-Cancer Drugs 26: 139–147.
Chen, Y.Y., Z.Q. Hu, T.Q. Hui, and H. Liu. 2020. Enhancer of zeste homolog 2 affects dental pulp inflammation by regulating macrophage chemotaxis. Beijing Da Xue Xue Bao. Yi Xue Ban 52: 18–23.
Kartenbeck, J., K. Schwechheimer, R. Moll, and W.W. Franke. 1984. Attachment of vimentin filaments to desmosomal plaques in human meningiomal cells and arachnoidal tissue. The Journal of Cell Biology 98: 1072–1081.
Yoshida, K., K. Sato, M. Tonogi, Y. Tanaka, G.Y. Yamane, and A. Katakura. 2015. Expression of cytokeratin 14 and 19 in process of oral carcinogenesis. The Bulletin of Tokyo Dental College 56: 105–111.
Krane, S.M. 1994. Clinical importance of metalloproteinases and their inhibitors. Annals of the New York Academy of Sciences 732: 1–10.
Birkedal-Hansen, H., W.G. Moore, M.K. Bodden, L.J. Windsor, B. Birkedal-Hansen, A. DeCarlo, et al. 1993. Matrix metalloproteinases: a review. Critical Reviews in Oral Biology and Medicine 4: 197–250.
Bergenholtz, G. 2000. Evidence for bacterial causation of adverse pulpal responses in resin-based dental restorations. Critical Reviews in Oral Biology and Medicine 11: 467–480.
Wahlgren, J., T. Salo, O. Teronen, H. Luoto, T. Sorsa, and L. Tjaderhane. 2002. Matrix metalloproteinase-8 (MMP-8) in pulpal and periapical inflammation and periapical root-canal exudates. International Endodontic Journal 35: 897–904.
Evrosimovska, B., C. Dimova, I. Kovacevska, and S. Panov. 2012. Concentration of collagenases (MMP-1, -8, -13) in patients with chronically inflamed dental pulp tissue. Prilozi 33: 191–204.
Rhim, E.M., S.J. Ahn, J.Y. Kim, K.H. Kim, H.W. Lee, E.C. Kim, K.Y. Kim, and S.H. Park. 2013. Stimulation of matrix metalloproteinases by tumor necrosis factor-alpha in human pulp cell cultures. Journal of Endodontia 39: 795–800.
Helder, M.N., A.L. Bronckers, and J.H. Woltgens. 1993. Dissimilar expression patterns for the extracellular matrix proteins osteopontin (OPN) and collagen type I in dental tissues and alveolar bone of the neonatal rat. Matrix 13: 415–425.
Luo, H., C. Wang, M. Liu, B. Yin, A. Peng, D. Huang, et al. 2018. Inhibition of SOX9 promotes inflammatory and immune responses of dental pulp. Journal of Endodontia 44: 792–799.
Goldberg, M., A. Njeh, and E. Uzunoglu. 2015. Is pulp inflammation a prerequisite for pulp healing and regeneration? Mediators of Inflammation 2015: 347649.
Cooper, P.R., M.J. Holder, and A.J. Smith. 2014. Inflammation and regeneration in the dentin-pulp complex: a double-edged sword. Journal of Endodontia 40: S46–S51.
Funding
This work was supported by the Natural Science Foundation of China (NSFC) (grant # 81800959).
Author information
Authors and Affiliations
Contributions
Jie He, Tianqian Hui, and Man Qin designed the study; Jie He and Yingyi Chen performed the research. Jie He, Yingyi Chen, and Ziqi Hu analyzed the data and contributed to the search and collation of literature. Jie He and Ling Ye wrote the manuscript. Ling Ye, Man Qin, and Tianqian Hui contributed to the revision of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The entire study was approved by the human research committee of Peking University School of Stomatology (ethics approval number: PKUSSIRB-201732003) and performed after written informed consent from patients was obtained. This study has been verified by the Peking University Biomedical Ethics Committee Approved by the Animal Welfare Ethics Committee (Approval Number: LA2018044).
Consent for publication
The participant has consented to the submission of the manuscript to the journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, J., Qin, M., Chen, Y. et al. EZH2 Promotes Extracellular Matrix Degradation via Nuclear Factor-κB (NF-κB) and p38 Signaling Pathways in Pulpitis. Inflammation 44, 1927–1936 (2021). https://doi.org/10.1007/s10753-021-01470-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01470-7