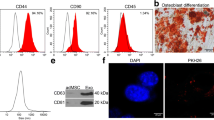
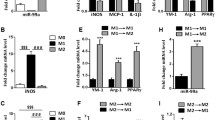
Abstract
Severe inflammation can lead to multiple organ dysfunction syndrome, which has high mortality. Adipose-derived stem cells have been shown to affect the inflammatory response of macrophages. However, the molecular mechanism of the anti-inflammatory capacity of adipose-derived stem cells (ADSCs) remains to be understood. In the present study, a macrophage inflammation model was established by LPS, and treated with different volumes of ADSC supernatant. Then, we investigated the key genes in the LPS group and treatment group by RT-PCR, RNA sequencing technology, and bioinformatics analysis. A total of 26 miRNAs and 11,882 mRNAs were differentially expressed between them. The expression of 15 of the miRNAs (9 upregulated and 6 downregulated) was confirmed by RT-PCR. GO and KEGG pathway analyses of the targets of the 9 significantly upregulated miRNAs showed that they were related to immune system process, inflammatory response, lipopolysaccharide, and TNF-α, NF-κB, Toll-like receptor, and MAPK signaling pathways. Moreover, a miRNA-mRNA network also revealed 8 important genes (Mapkapk2, Sepp1, Cers6, Snn, ZfP568, Ccdc93, Pofut1, Pik3cd). We finally confirmed the expression of these 8 targeted genes by performing the RT-PCR analysis. This study may provide a new understanding of the molecular mechanism of ADSCs in the inflammatory response related to multiple miRNAs and mRNAs.









Similar content being viewed by others
Data Availability
All data generated and/or analyzed during this study are included in this published article.
Abbreviations
- ADSCs:
-
adipose tissue–derived stem cells
- ADSC-sup:
-
adipose tissue–derived stem cell supernatant
- BMDM:
-
bone marrow–derived macrophage
- BSA:
-
albumin bovine serum
- DE:
-
differentially expressed
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBS:
-
fetal bovine serum
- IL:
-
interleukin
- LPS:
-
lipopolysaccharide
- MSC:
-
mesenchymal stem cell
- PBS:
-
phosphate-buffered saline
- RNA-seq:
-
RNA sequencing
- RT-PCR:
-
reverse transcription polymerase chain reaction
- TNF:
-
tumor necrosis factor
References
Broom, L.J., and M.H. Kogut. 2018. Inflammation: friend or foe for animal production? Poultry Science 97: 510–514.
Matthay, M.A., S. Pati, and J.W. Lee. 2017. Concise review: mesenchymal stem (stromal) cells: biology and preclinical evidence for therapeutic potential for organ dysfunction following trauma or sepsis. Stem Cells 35: 316–324.
Zheng, D., Y. Yu, M. Li, G. Wang, R. Chen, G.C. Fan, C. Martin, S. Xiong, and T. Peng. 2016. Inhibition of microRNA 195 prevents apoptosis and multiple-organ injury in mouse models of sepsis. The Journal of Infectious Diseases 213: 1661–1670.
Qiu, G., G. Zheng, M. Ge, L. Huang, H. Tong, P. Chen, D. Lai, Y. Hu, B. Cheng, Q. Shu, and J. Xu. 2017. Adipose-derived mesenchymal stem cells modulate CD14(++)CD16(+) expression on monocytes from sepsis patients in vitro via prostaglandin E2. Stem Cell Research & Therapy 8: 97.
Kawanishi, N., H. Yano, Y. Yokogawa, and K. Suzuki. 2010. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exercise Immunology Review 16: 105–118.
Wang, Y., G.Z. Wang, P.S. Rabinovitch, and I. Tabas. 2014. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-kappaB-mediated inflammation in macrophages. Circulation Research 114: 421–433.
Ti, D., H. Hao, C. Tong, J. Liu, L. Dong, J. Zheng, Y. Zhao, H. Liu, X. Fu, and W. Han. 2015. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. Journal of Translational Medicine 13: 308.
Ivanova, E.A., and A.N. Orekhov. 2016. Monocyte activation in immunopathology: cellular test for development of diagnostics and therapy. Journal of Immunology Research 2016: 4789279.
Li, Y., Y. Wu, X. Yao, F. Hao, C. Yu, Y. Bao, et al. 2017. Ginkgolide A ameliorates LPS-induced inflammatory responses in vitro and in vivo. International Journal of Molecular Sciences: 18.
Xu, Q., M. Liu, Q. Liu, W. Wang, Y. Du, and H. Yin. 2017. The inhibition of LPS-induced inflammation in RAW264.7 macrophages via the PI3K/Akt pathway by highly N-acetylated chitooligosaccharide. Carbohydrate Polymers 174: 1138–1143.
Varela, M.L., M. Mogildea, I. Moreno, and A. Lopes. 2018. Acute inflammation and metabolism. Inflammation 41: 1115–1127.
Zhao, H., Q. Shang, Z. Pan, Y. Bai, Z. Li, H. Zhang, Q. Zhang, C. Guo, L. Zhang, and Q. Wang. 2018. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes 67: 235–247.
Sukho, P., A. Cohen, J.W. Hesselink, J. Kirpensteijn, F. Verseijden, and Y.M. Bastiaansen-Jenniskens. 2018. Adipose tissue-derived stem cell sheet application for tissue healing in vivo: a systematic review. Tissue Engineering. Part B, Reviews 24: 37–52.
Yu, H.S., M.K. Park, S.A. Kang, K.S. Cho, S.J. Mun, and H.J. Roh. 2017. Culture supernatant of adipose stem cells can ameliorate allergic airway inflammation via recruitment of CD4(+)CD25(+)Foxp3 T cells. Stem Cell Research & Therapy 8: 8.
Oses, C., B. Olivares, M. Ezquer, C. Acosta, P. Bosch, M. Donoso, P. Léniz, and F. Ezquer. 2017. Preconditioning of adipose tissue-derived mesenchymal stem cells with deferoxamine increases the production of pro-angiogenic, neuroprotective and anti-inflammatory factors: potential application in the treatment of diabetic neuropathy. PLoS One 12: e0178011.
Song, W.J., Q. Li, M.O. Ryu, J.O. Ahn, B.D. Ha, J.Y. Chan, et al. 2017. TSG-6 secreted by human adipose tissue-derived mesenchymal stem cells ameliorates DSS-induced colitis by inducing M2 macrophage polarization in mice. Scientific Reports 7: 5187.
Kawata, Y., A. Tsuchiya, S. Seino, Y. Watanabe, Y. Kojima, S. Ikarashi, K. Tominaga, J. Yokoyama, S. Yamagiwa, and S. Terai. 2019. Early injection of human adipose tissue-derived mesenchymal stem cell after inflammation ameliorates dextran sulfate sodium-induced colitis in mice through the induction of M2 macrophages and regulatory T cells. Cell and Tissue Research 376: 257–271.
Pedrazza, L., M. Cubillos-Rojas, F.C.D. Mesquita, C. Luft, A.A. Cunha, J.L. Rosa, et al. 2017. Mesenchymal stem cells decrease lung inflammation during sepsis, acting through inhibition of the MAPK pathway. Stem Cell Research & Therapy 8: 289.
Cao, M., W. Zhang, J. Li, J. Zhang, L. Li, M. Liu, et al. 2018. Inhibition of SIRT1 by microRNA-9, the key point in process of LPS-induced severe inflammation. Archives of Biochemistry and Biophysics.
Essandoh, K., Y. Li, J. Huo, and G.C. Fan. 2016. MiRNA-mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock 46: 122–131.
Czimmerer, Z., A. Horvath, B. Daniel, G. Nagy, I. Cuaranta-Monroy, M. Kiss, Z. Kolostyak, S. Poliska, L. Steiner, N. Giannakis, T. Varga, and L. Nagy. 2018. Dynamic transcriptional control of macrophage miRNA signature via inflammation responsive enhancers revealed using a combination of next generation sequencing-based approaches. Biochim Biophys Acta Gene Regul Mech 1861: 14–28.
Zhao, J., X. Li, J. Hu, F. Chen, S. Qiao, X. Sun, L. Gao, J. Xie, and B. Xu. 2019. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovascular Research 115: 1205–1216.
Li, X., L. Liu, J. Yang, Y. Yu, J. Chai, L. Wang, L. Ma, and H. Yin. 2016. Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR-181c attenuating burn-induced excessive inflammation. Ebiomedicine 8: 72–82.
Tang, G.N., C.L. Li, Y. Yao, Z.B. Xu, M.X. Deng, S.Y. Wang, Y.Q. Sun, J.B. Shi, and Q.L. Fu. 2016. MicroRNAs involved in asthma after mesenchymal stem cells treatment. Stem Cells and Development 25: 883–896.
Bai, X., T. He, Y. Liu, J. Zhang, X. Li, J. Shi, K. Wang, F. Han, W. Zhang, Y. Zhang, W. Cai, and D. Hu. 2018. Acetylation-dependent regulation of notch signaling in macrophages by SIRT1 affects sepsis development. Frontiers in Immunology 9: 762.
Zhang, Y.Y., H.B. Wang, Y.N. Wang, H.C. Wang, S. Zhang, J.Y. Hong, H.F. Guo, D. Chen, Y. Yang, and L.S. Zan. 2017. Transcriptome analysis of mRNA and microRNAs in intramuscular fat tissues of castrated and intact male Chinese Qinchuan cattle. PLoS One 12: e0185961.
Shojafar, E., M. Soleimani Mehranjani, and S.M.A. Shariatzadeh. 2019. Adipose derived mesenchymal stem cells improve the structure and function of autografted mice ovaries through reducing oxidative stress and inflammation: a stereological and biochemical analysis. Tissue and Cell 56: 23–30.
Zhao, H., Q. Shang, Z. Pan, Y. Bai, Z. Li, H. Zhang, et al. 2018. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissues. Diabetes 67: db170356.
Sangwoon, C., R. Ravi, L.Y. Gyu, P. Gye Young, K. Manjula, D. Jing, et al. 2015. Distinct role of FoxO1 in M-CSF- and GM-CSF-differentiated macrophages contributes LPS-mediated IL-10: implication in hyperglycemia. Journal of Leukocyte Biology 97: 327–339.
Lalani, A.I., C.R. Moore, L. Chang, B.Z. Kreider, L. Yan, H.C. Morse, et al. 2015. Myeloid cell TRAF3 regulates immune responses and inhibits inflammation and tumor development in mice. Journal of Immunology 194: 334–348.
Nahid, M.A., Y. Bing, P.R. Dominguez-Gutierrez, K. Lakshmyya, S. Minoru, and E.K.L. Chan. 2013. Regulation of TLR2-mediated tolerance and cross-tolerance through IRAK4 modulation by miR-132 and miR-212. Journal of Immunology 190: 1250–1263.
Muraleedharan, C.K., S.A. Mcclellan, S.A. Ekanayaka, R. Francis, A. Zmejkoski, L.D. Hazlett, et al. 2019. The miR-183/96/182 cluster regulates macrophage functions in response to Pseudomonas aeruginosa. Journal of innate immunity.
Guilbaud, M., C. Gentil, C. Peccate, E. Gargaun, I. Holtzmann, C. Gruszczynski, et al. 2018. miR-708-5p and miR-34c-5p are involved in nNOS regulation in dystrophic context. Skeletal Muscle 8: 15.
Liu, Y., Y. Liu, M. Han, X. Du, X. Liu, Q. Zhang, et al. 2019. Edwardsiella tarda-induced miR-7a functions as a suppressor in PI3K/AKT/GSK3beta signaling pathway by targeting insulin receptor substrate-2 (IRS2a and IRS2b) in Paralichthys olivaceus. Fish & Shellfish Immunology.
Chen, W., X. Ma, P. Zhang, Q. Li, X. Liang, and J. Liu. 2017. MiR-212-3p inhibits LPS-induced inflammatory response through targeting HMGB1 in murine macrophages. Experimental Cell Research 350: 318–326.
Fen, L., L. Yong, J. Rong, N. Cheng, Z. Zhenguo, Z. Ning, et al. 2015. miR-132 inhibits lipopolysaccharide-induced inflammation in alveolar macrophages by the cholinergic anti-inflammatory pathway. Experimental Lung Research 41: 261–269.
Soni, S., P. Anand, and Y.S. Padwad. 2019. MAPKAPK2: the master regulator of RNA-binding proteins modulates transcript stability and tumor progression. Journal of Experimental & Clinical Cancer Research 38: 121.
Phinney, B.B., A.L. Ray, A.S. Peretti, S.J. Jerman, C. Grim, I.V. Pinchuk, and E.J. Beswick. 2018. MK2 regulates macrophage chemokine activity and recruitment to promote colon tumor growth. Frontiers in Immunology 9: 1857.
Ehlting, C., N. Ronkina, O. Bohmer, U. Albrecht, K.A. Bode, K.S. Lang, et al. 2011. Distinct functions of the mitogen-activated protein kinase-activated protein (MAPKAP) kinases MK2 and MK3: MK2 mediates lipopolysaccharide-induced signal transducers and activators of transcription 3 (STAT3) activation by preventing negative regulatory effects of MK3. The Journal of Biological Chemistry 286: 24113–24124.
Fiore, M., S. Forli, and F. Manetti. 2015. Targeting mitogen-activated protein kinase-activated protein kinase 2 (MAPKAPK2, MK2): medicinal chemistry efforts to lead small molecule inhibitors to clinical trials. Journal of Medicinal Chemistry 59: 3609.
Barrett, C.W., V.K. Reddy, S.P. Short, A.K. Motley, M.K. Lintel, A.M. Bradley, T. Freeman, J. Vallance, W. Ning, B. Parang, S.V. Poindexter, B. Fingleton, X. Chen, M.K. Washington, K.T. Wilson, N.F. Shroyer, K.E. Hill, R.F. Burk, and C.S. Williams. 2015. Selenoprotein P influences colitis-induced tumorigenesis by mediating stemness and oxidative damage. Journal of Clinical Investigation 125: 2646–2660.
Solinas, G., S.M. Schiarea, M. Fabbri, S. Pesce, L. Zammataro, F. Pasqualini, et al. 2010. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for m2-polarization, influencing tumor cell motility. Journal of Immunology 185: 642–652.
Kim, M.H., H.K. Ahn, E.J. Lee, S.J. Kim, Y.R. Kim, J.W. Park, and W.J. Park. 2017. Hepatic inflammatory cytokine production can be regulated by modulating sphingomyelinase and ceramide synthase 6. International Journal of Molecular Medicine 39: 453–462.
Scheffel, M.J., K. Helke, P. Lu, J.S. Bowers, B. Ogretmen, E. Garrett-Mayer, C.M. Paulos, and C. Voelkel-Johnson. 2017. Adoptive transfer of ceramide synthase 6 deficient splenocytes reduces the development of colitis. Scientific Reports 7: 15552.
Maho, S., K.E. Blauvelt, K.F. Liem, and M.J. García-García. 2011. TRIM28 is required by the mouse KRAB domain protein ZFP568 to control convergent extension and morphogenesis of extra-embryonic tissues. Development 138: 5333–5343.
Phillips-Krawczak, C.A., A. Singla, P. Starokadomskyy, Z. Deng, D.G. Osborne, H. Li, C.J. Dick, T.S. Gomez, M. Koenecke, J.S. Zhang, H. Dai, L.F. Sifuentes-Dominguez, L.N. Geng, S.H. Kaufmann, M.Y. Hein, M. Wallis, J. McGaughran, J. Gecz, B. Sluis, D.D. Billadeau, and E. Burstein. 2015. COMMD1 is linked to the WASH complex and regulates endosomal trafficking of the copper transporter ATP7A. Molecular Biology of the Cell 26: 91–103.
Ming, H., L. Zhexuan, and Z. Yuan. 2013. The alteration of copper homeostasis in inflammation induced by lipopolysaccharides. Biological Trace Element Research 154: 268–274.
Takeda, A.J., Y. Zhang, G.L. Dornan, B.D. Siempelkamp, M.L. Jenkins, H.F. Matthews, J.J. McElwee, W. Bi, F.O. Seeborg, H.C. Su, J.E. Burke, and C.L. Lucas. 2017. Novel PIK3CD mutations affecting N-terminal residues of p110δ cause activated PI3Kδ syndrome (APDS) in humans. Journal of Allergy & Clinical Immunology 140: 1152–1156.e10.
Wan, G., L. Tian, Y. Yu, F. Li, X. Wang, C. Li, et al. 2017. Overexpression of Pofut1 and activated Notch1 may be associated with poor prognosis in breast cancer. Biochemical & Biophysical Research Communications 491.
Funding
This work was supported by the National Natural Science Foundation of China (81501663 and 81530064).
Author information
Authors and Affiliations
Contributions
XB, TH, and ML contribute equally as first authors. XB and DH designed the project. ML, LL, MC, and YL performed most of the experiments. JC and ML analyzed data. CY verified cells and built the macrophage inflammation model. CY, TK, and WJ contributed to results’ analysis. XB, JH, and DH revised the manuscript and approved the final submission.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethics Approval
This study was carried out in accordance with the principles of ARRIVE approved by the Ethics Committee of Xijing Hospital, affiliated with the Fourth Military Medical University (No.: XJYYLL-2015206).
Consent to Participate
Written informed consent was obtained from individual or guardian participants.
Consent for Publication
Not applicable.
Code Availability
Not applicable
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Bai, X., He, T., Liu, M. et al. Integrative Analysis of MicroRNAs and mRNAs in LPS-Induced Macrophage Inflammation Based on Adipose Tissue Stem Cell Therapy. Inflammation 44, 407–420 (2021). https://doi.org/10.1007/s10753-020-01345-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01345-3